Abstract
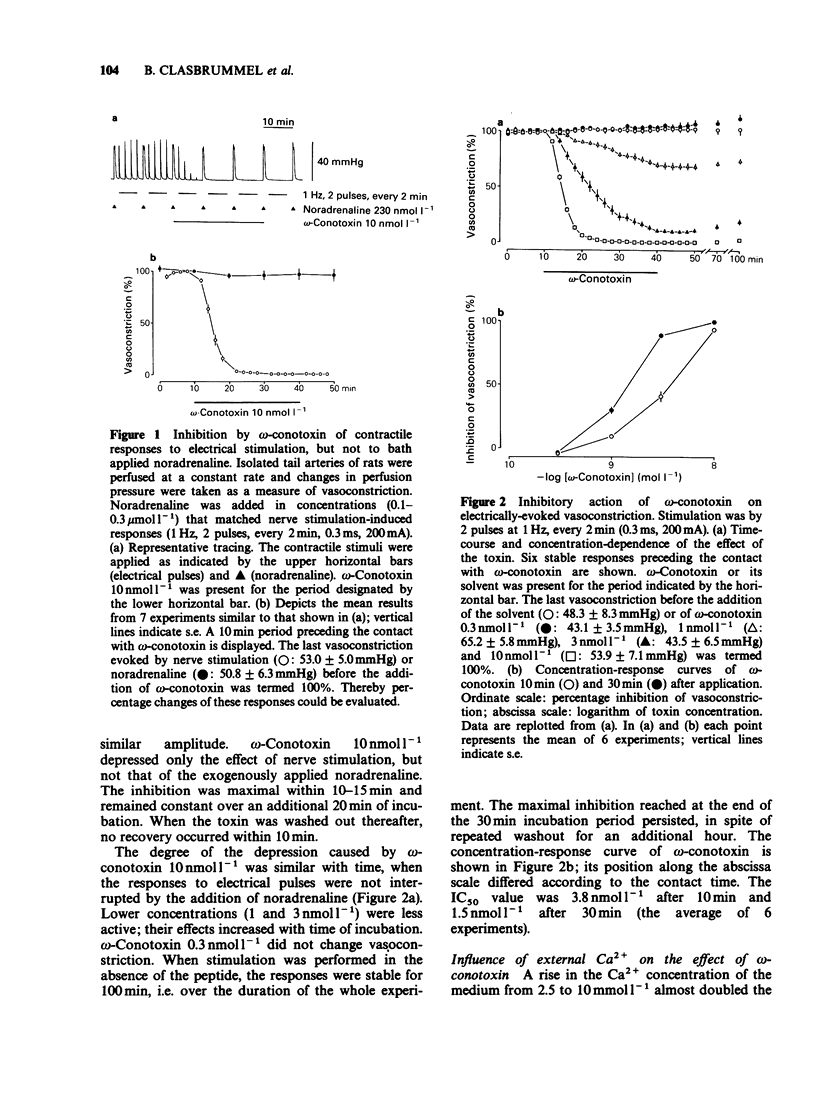
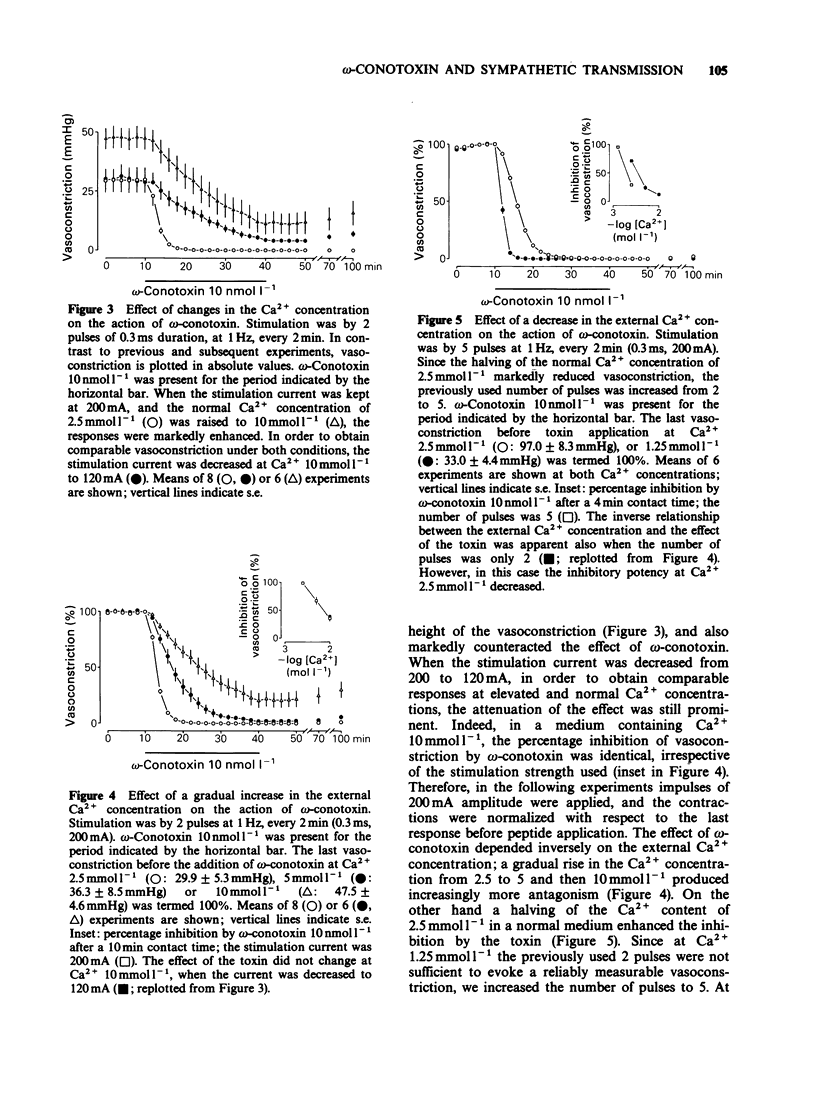
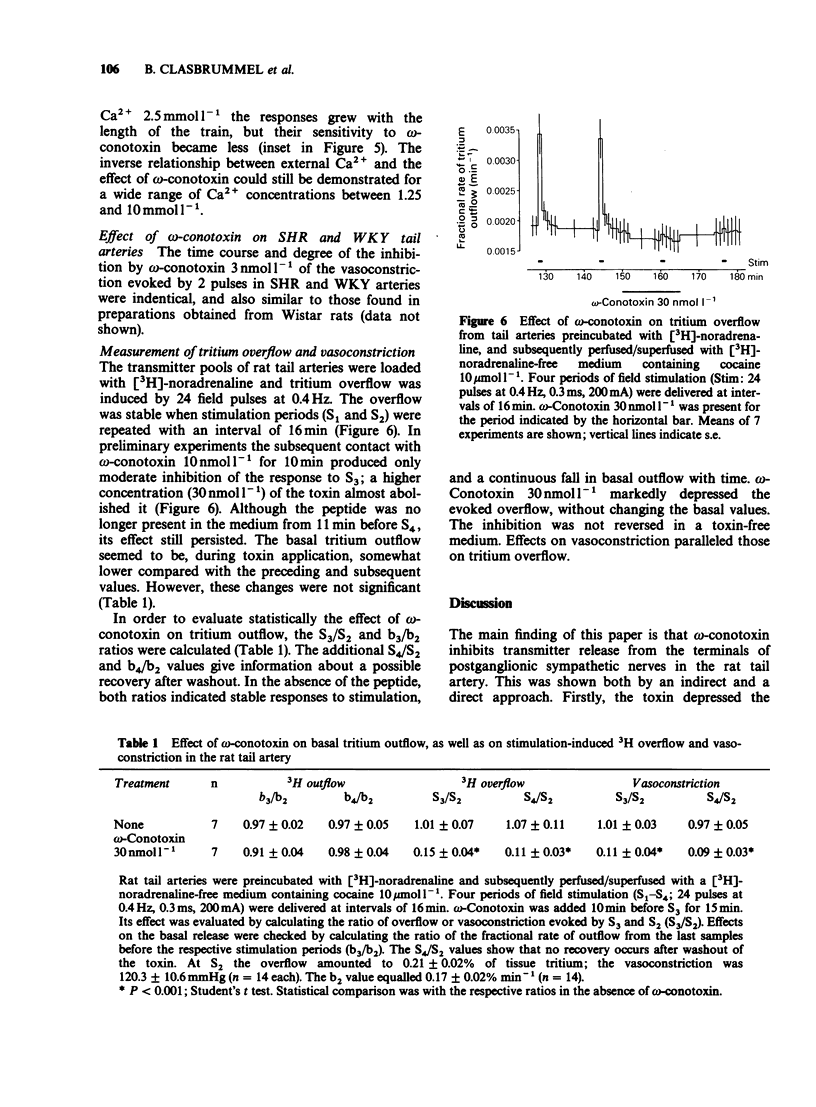
1. The perivascular nerves of isolated tail arteries from Wistar rats were stimulated with field pulses (1 Hz, 2 pulses, every 2 min). omega-Conotoxin 10 nmol l-1 depressed neurogenically mediated contractions, but did not influence the contractions to noradrenaline 0.1-0.3 mumol l-1. 2. The inhibitory effect of omega-conotoxin was concentration-dependent (IC50 = 3.8 nmol l-1). It did not reach a steady-state during 30 min incubation and could not be reversed upon subsequent washout for another 60 min. 3. A gradual increase in the Ca2+ concentration of the medium from 1.25 mmol l-1 to 10 mmol l-1 enhanced vasoconstriction and attenuated the action of omega-conotoxin 10 nmol l-1. When a low stimulation intensity (120 mA) was used at high external Ca2+ (10 mmol l-1), similar contractile responses were obtained as under normal conditions (200 mA current, 2.5 mmol l-1 Ca2+). However, the inverse relationship between the effect of the toxin and external Ca2+ remained unchanged. 4. The time-course and degree of the inhibition by omega-conotoxin 3 nmol l-1 was identical in tail arteries of spontaneously hypertensive rats (SHR) and their normotensive controls (WKY). 5. When tail arteries of Wistar rats were preincubated with [3H]-noradrenaline, field stimulation (0.4 Hz, 24 pulses, every 16 min) evoked tritium overflow and vasoconstriction. omega-Conotoxin 30 nmol l-1 inhibited both responses to a similar extent. 6. Our results suggest that omega-conotoxin selectively blocks Ca2+ channels in the terminals of perivascular nerves and thereby reduces the release, but not the contractile effect of the sympathetic transmitter.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Koyano K., Saisu H., Nishiuchi Y., Sakakibara S. Binding of omega-conotoxin to receptor sites associated with the voltage-sensitive calcium channel. Neurosci Lett. 1986 Nov 11;71(2):203–208. doi: 10.1016/0304-3940(86)90559-8. [DOI] [PubMed] [Google Scholar]
- Bou J., Llenas J., Massingham R. Calcium entry blocking drugs, 'calcium antagonists' and vascular smooth muscle function. J Auton Pharmacol. 1983 Sep;3(3):219–232. doi: 10.1111/j.1474-8673.1983.tb00538.x. [DOI] [PubMed] [Google Scholar]
- Bucher B., Bettermann R., Illes P. Plasma concentration and vascular effect of beta-endorphin in spontaneously hypertensive and Wistar Kyoto rats. Naunyn Schmiedebergs Arch Pharmacol. 1987 Apr;335(4):428–432. doi: 10.1007/BF00165558. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Warland J. J. A pharmacological study of the rabbit saphenous artery in vitro: a vessel with a large purinergic contractile response to sympathetic nerve stimulation. Br J Pharmacol. 1987 Jan;90(1):111–120. doi: 10.1111/j.1476-5381.1987.tb16830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Catecholamine action on smooth muscle. Pharmacol Rev. 1987 Mar;39(1):49–96. [PubMed] [Google Scholar]
- Ceña V., García A. G., Khoyi M. A., Salaices M., Sanchez-García P. Effect of the dihydropyridine Bay K 8644 on the release of [3H]-noradrenaline from the rat isolated vas deferens. Br J Pharmacol. 1985 Jul;85(3):691–696. doi: 10.1111/j.1476-5381.1985.tb10565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz L. J., Johnson D. S., Olivera B. M. Characterization of the omega-conotoxin target. Evidence for tissue-specific heterogeneity in calcium channel types. Biochemistry. 1987 Feb 10;26(3):820–824. doi: 10.1021/bi00377a024. [DOI] [PubMed] [Google Scholar]
- Cruz L. J., Olivera B. M. Calcium channel antagonists. Omega-conotoxin defines a new high affinity site. J Biol Chem. 1986 May 15;261(14):6230–6233. [PubMed] [Google Scholar]
- Dooley D. J., Lupp A., Hertting G. Inhibition of central neurotransmitter release by omega-conotoxin GVIA, a peptide modulator of the N-type voltage-sensitive calcium channel. Naunyn Schmiedebergs Arch Pharmacol. 1987 Oct;336(4):467–470. doi: 10.1007/BF00164885. [DOI] [PubMed] [Google Scholar]
- Dooley D. J., Lupp A., Hertting G., Osswald H. Omega-conotoxin GVIA and pharmacological modulation of hippocampal noradrenaline release. Eur J Pharmacol. 1988 Mar 29;148(2):261–267. doi: 10.1016/0014-2999(88)90572-9. [DOI] [PubMed] [Google Scholar]
- Drew G. M. The effect of different calcium concentrations on the inhibitory effect of presynaptic alpha-adrenoceptors in the rat vas deferens. Br J Pharmacol. 1978 Jul;63(3):417–419. doi: 10.1111/j.1476-5381.1978.tb07792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey M. P., Muscholl E., Pfeiffer A. Muscarinic inhibition of potassium-induced noradrenaline release and its dependence on the calcium concentration. Naunyn Schmiedebergs Arch Pharmacol. 1975;291(1):1–15. doi: 10.1007/BF00510816. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway M. P., Westfall T. C. The release of endogenous norepinephrine from the coccygeal artery of spontaneously hypertensive and Wistar-Kyoto rats. Circ Res. 1982 Aug;51(2):225–232. doi: 10.1161/01.res.51.2.225. [DOI] [PubMed] [Google Scholar]
- Godfraind T., Miller R., Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986 Dec;38(4):321–416. [PubMed] [Google Scholar]
- Göthert M., Nawroth P., Neumeyer H. Inhibitory effects of verapamil, prenylamine and D 600 on Ca2+-dependent noradrenaline release from the sympathetic nerves of isolated rabbit hearts. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(1):11–19. doi: 10.1007/BF00499869. [DOI] [PubMed] [Google Scholar]
- Hicks P. E., Tierney C., Langer S. Z. Preferential antagonism by diltiazem of alpha 2-adrenoceptor mediated vasoconstrictor responses in perfused tail arteries of spontaneous hypertensive rats. Naunyn Schmiedebergs Arch Pharmacol. 1985 Feb;328(4):388–395. doi: 10.1007/BF00692906. [DOI] [PubMed] [Google Scholar]
- Illes P., Bettermann R., Brod I., Bucher B. Beta-endorphin-sensitive opioid receptors in the rat tail artery. Naunyn Schmiedebergs Arch Pharmacol. 1987 Apr;335(4):420–427. doi: 10.1007/BF00165557. [DOI] [PubMed] [Google Scholar]
- Illes P. Mechanisms of receptor-mediated modulation of transmitter release in noradrenergic, cholinergic and sensory neurones. Neuroscience. 1986 Apr;17(4):909–928. doi: 10.1016/0306-4522(86)90071-0. [DOI] [PubMed] [Google Scholar]
- Illes P., Zieglgänsberger W., Herz A. Calcium reverses the inhibitory action of morphine on neuroeffector transmission in the mouse vas deferens. Brain Res. 1980 Jun 9;191(2):511–522. doi: 10.1016/0006-8993(80)91299-8. [DOI] [PubMed] [Google Scholar]
- Karaki H., Nakagawa H., Urakawa N. Effects of calcium antagonists on release of [3H]noradrenaline in rabbit aorta. Eur J Pharmacol. 1984 Jun 1;101(3-4):177–183. doi: 10.1016/0014-2999(84)90154-7. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Saville V. L., Burnstock G. The contributions of noradrenaline and ATP to the responses of the rabbit central ear artery to sympathetic nerve stimulation depend on the parameters of stimulation. Eur J Pharmacol. 1986 Apr 2;122(3):291–300. doi: 10.1016/0014-2999(86)90409-7. [DOI] [PubMed] [Google Scholar]
- Kerr L. M., Yoshikami D. A venom peptide with a novel presynaptic blocking action. Nature. 1984 Mar 15;308(5956):282–284. doi: 10.1038/308282a0. [DOI] [PubMed] [Google Scholar]
- Knaus H. G., Striessnig J., Koza A., Glossmann H. Neurotoxic aminoglycoside antibiotics are potent inhibitors of [125I]-Omega-Conotoxin GVIA binding to guinea-pig cerebral cortex membranes. Naunyn Schmiedebergs Arch Pharmacol. 1987 Nov;336(5):583–586. doi: 10.1007/BF00169318. [DOI] [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D. H., Cruz L. J., Olivera B. M., Tsien R. W., Yoshikami D. Omega-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medgett I. C., Hicks P. E., Langer S. Z. Smooth muscle alpha-2 adrenoceptors mediate vasoconstrictor responses to exogenous norepinephrine and to sympathetic stimulation to a greater extent in spontaneously hypertensive than in Wistar Kyoto rat tail arteries. J Pharmacol Exp Ther. 1984 Oct;231(1):159–165. [PubMed] [Google Scholar]
- Medgett I. C., Langer S. Z. Heterogeneity of smooth muscle alpha adrenoceptors in rat tail artery in vitro. J Pharmacol Exp Ther. 1984 Jun;229(3):823–830. [PubMed] [Google Scholar]
- Medgett I. C., Rajanayagam M. A. Effects of reduced calcium ion concentration and of diltiazem on vasoconstrictor responses to noradrenaline and sympathetic nerve stimulation in rat isolated tail artery. Br J Pharmacol. 1984 Dec;83(4):889–898. doi: 10.1111/j.1476-5381.1984.tb16528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J., Freedman S. B. Are dihydropyridine binding sites voltage sensitive calcium channels? Life Sci. 1984 Mar 26;34(13):1205–1221. doi: 10.1016/0024-3205(84)90543-5. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Mochida S., Kobayashi H. Effects of Ca antagonists on the action potential and their relationship to the muscarinic ACh actions in isolated sympathetic neurons of rabbits. Neurosci Lett. 1986 Dec 12;72(2):205–210. doi: 10.1016/0304-3940(86)90081-9. [DOI] [PubMed] [Google Scholar]
- Muramatsu I. Evidence for sympathetic, purinergic transmission in the mesenteric artery of the dog. Br J Pharmacol. 1986 Mar;87(3):478–480. doi: 10.1111/j.1476-5381.1986.tb10187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachshen D. A. Selectivity of the Ca binding site in synaptosome Ca channels. Inhibition of Ca influx by multivalent metal cations. J Gen Physiol. 1984 Jun;83(6):941–967. doi: 10.1085/jgp.83.6.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Gray W. R., Zeikus R., McIntosh J. M., Varga J., Rivier J., de Santos V., Cruz L. J. Peptide neurotoxins from fish-hunting cone snails. Science. 1985 Dec 20;230(4732):1338–1343. doi: 10.1126/science.4071055. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., McIntosh J. M., Cruz L. J., Luque F. A., Gray W. R. Purification and sequence of a presynaptic peptide toxin from Conus geographus venom. Biochemistry. 1984 Oct 23;23(22):5087–5090. doi: 10.1021/bi00317a001. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Wagner J. A., Snyder S. H., Thayer S. A., Olivera B. M., Miller R. J. Brain voltage-sensitive calcium channel subtypes differentiated by omega-conotoxin fraction GVIA. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8804–8807. doi: 10.1073/pnas.83.22.8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier J., Galyean R., Gray W. R., Azimi-Zonooz A., McIntosh J. M., Cruz L. J., Olivera B. M. Neuronal calcium channel inhibitors. Synthesis of omega-conotoxin GVIA and effects on 45Ca uptake by synaptosomes. J Biol Chem. 1987 Jan 25;262(3):1194–1198. [PubMed] [Google Scholar]
- Sano K., Enomoto K., Maeno T. Effects of synthetic omega-conotoxin, a new type Ca2+ antagonist, on frog and mouse neuromuscular transmission. Eur J Pharmacol. 1987 Sep 11;141(2):235–241. doi: 10.1016/0014-2999(87)90268-8. [DOI] [PubMed] [Google Scholar]
- Vidal M., Hicks P. E., Langer S. Z. Differential effects of alpha-beta-methylene ATP on responses to nerve stimulation in SHR and WKY tail arteries. Naunyn Schmiedebergs Arch Pharmacol. 1986 Apr;332(4):384–390. doi: 10.1007/BF00500092. [DOI] [PubMed] [Google Scholar]
- Vizi E. S. Presynaptic modulation of neurochemical transmission. Prog Neurobiol. 1979;12(3-4):181–290. doi: 10.1016/0301-0082(79)90011-x. [DOI] [PubMed] [Google Scholar]
- Wakade A. R., Wakade T. D. Inhibition of noradrenaline release by adenosine. J Physiol. 1978 Sep;282:35–49. doi: 10.1113/jphysiol.1978.sp012446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall T. C., Meldrum M. J. Alterations in the release of norepinephrine at the vascular neuroeffector junction in hypertension. Annu Rev Pharmacol Toxicol. 1985;25:621–641. doi: 10.1146/annurev.pa.25.040185.003201. [DOI] [PubMed] [Google Scholar]
- Zelis R., Wichmann T., Starke K. Inhibition by diltiazem of norepinephrine release from sympathetic nerves in the rabbit pulmonary artery. Pharmacology. 1985;31(5):268–277. doi: 10.1159/000138131. [DOI] [PubMed] [Google Scholar]
- Zsotér T. T., Wolchinsky C., Endrenyi L. Effects of verapamil on [3H]norepinephrine release. J Cardiovasc Pharmacol. 1984 Nov-Dec;6(6):1060–1066. [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline and adenosine triphosphate as co-transmitters of neurogenic vasoconstriction in rabbit mesenteric artery. J Physiol. 1985 Oct;367:435–455. doi: 10.1113/jphysiol.1985.sp015834. [DOI] [PMC free article] [PubMed] [Google Scholar]


