Abstract
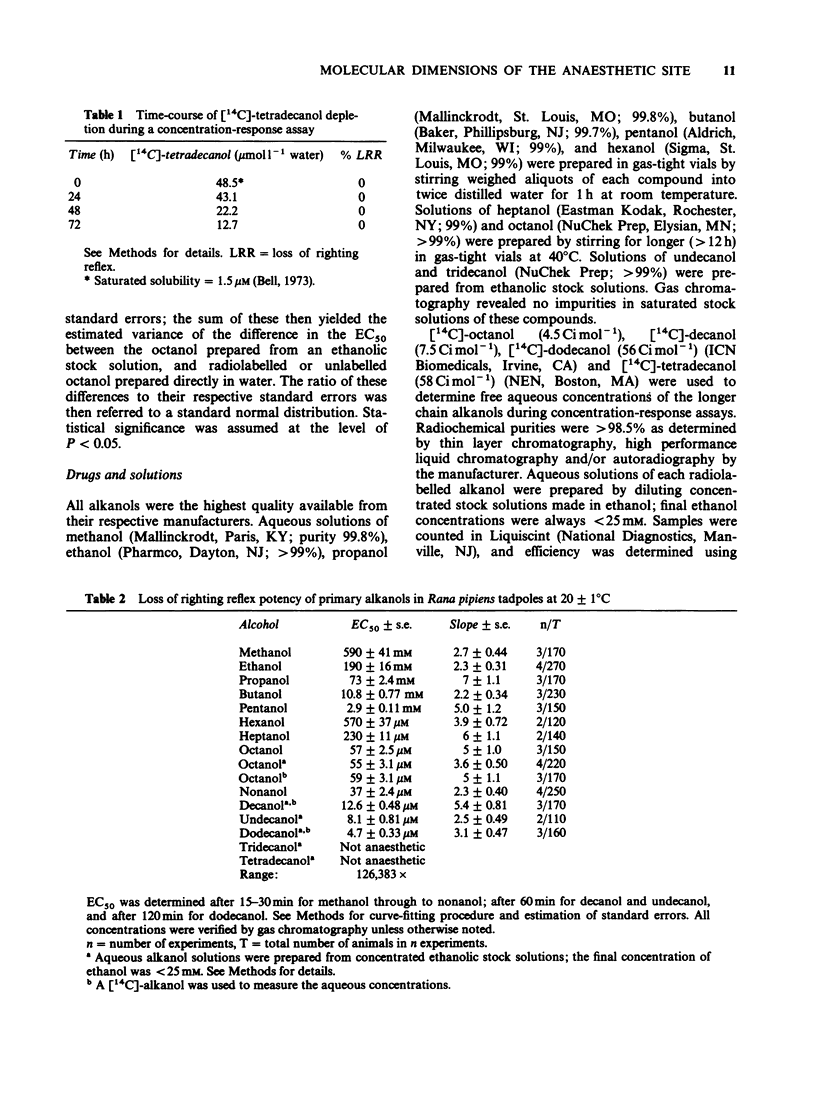
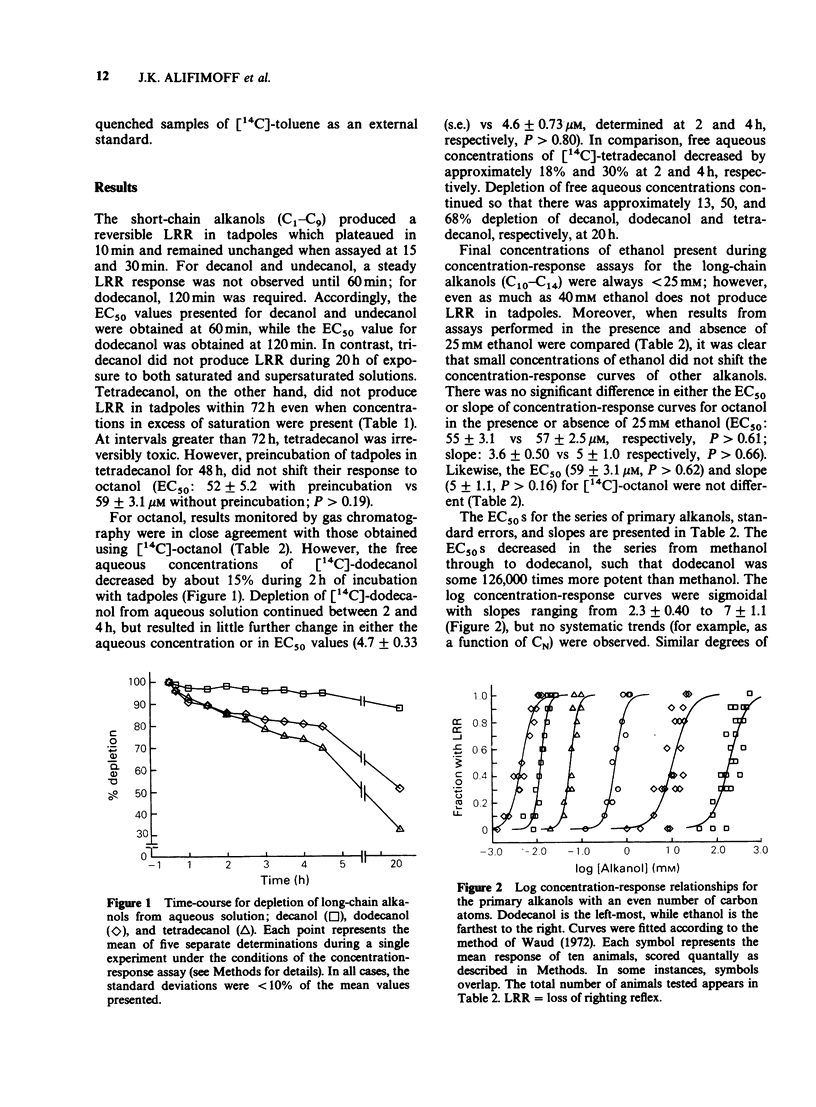
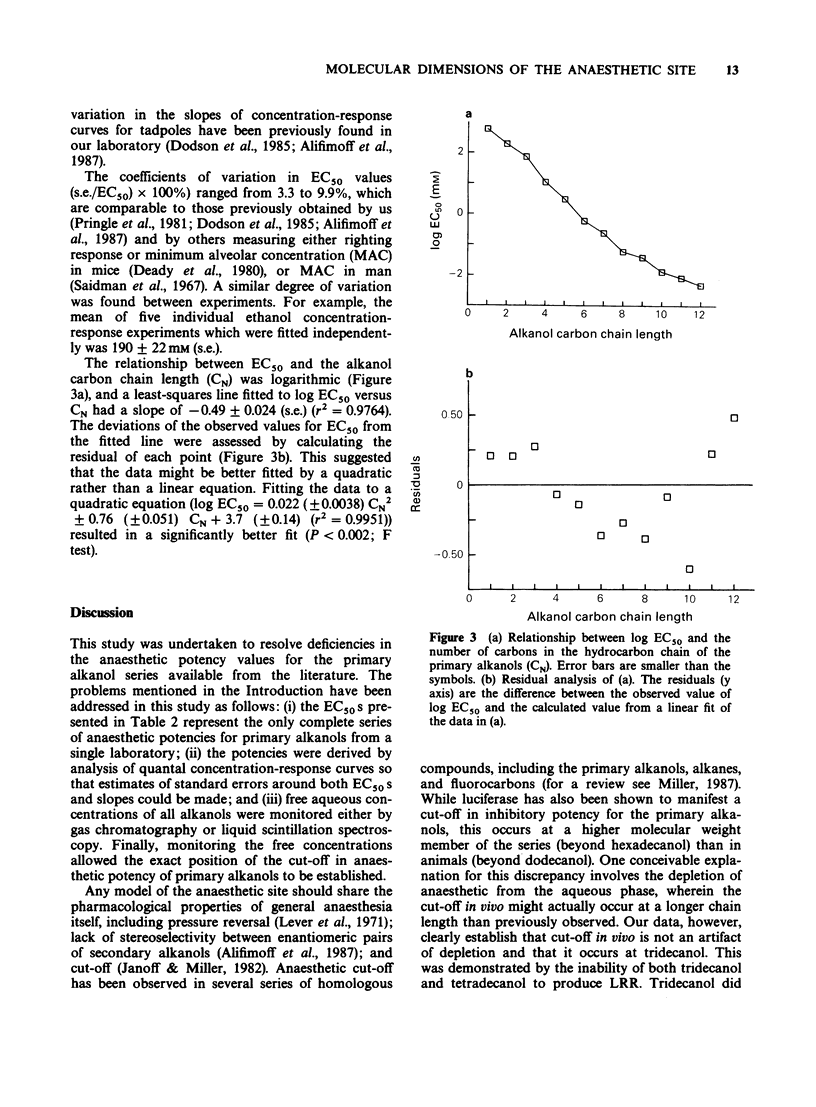
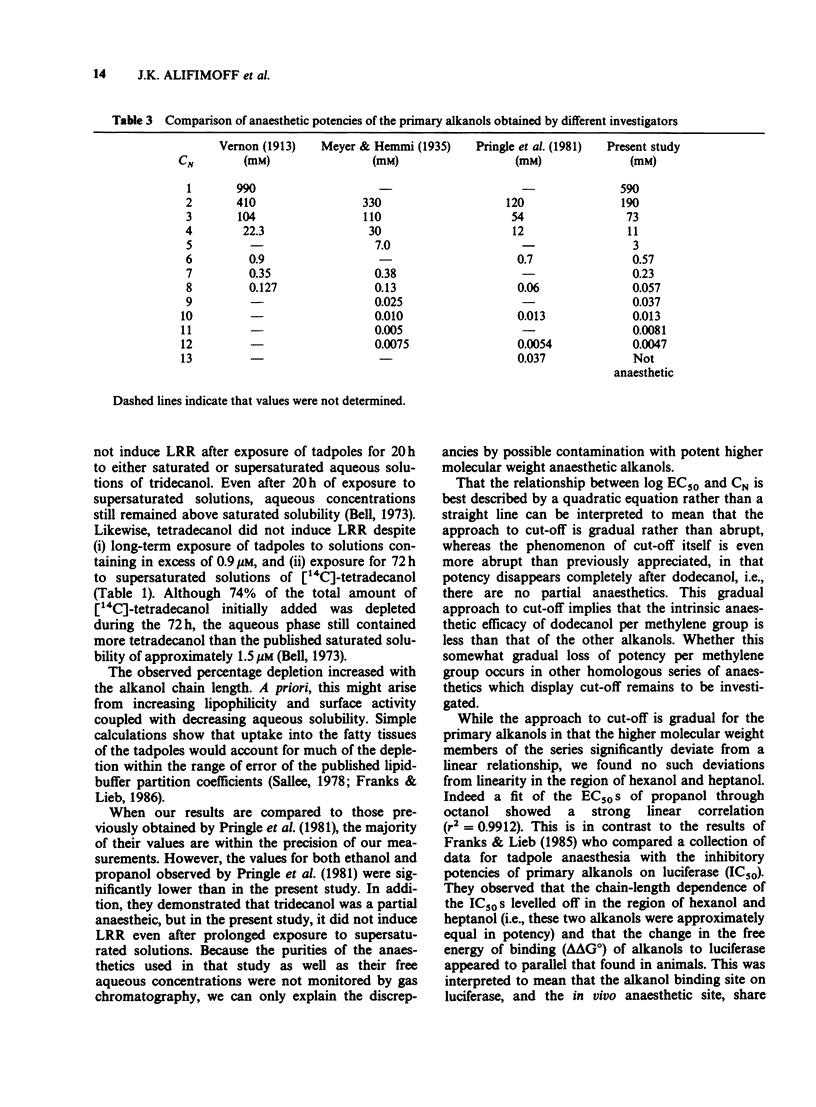
1. We have redetermined the anaesthetic potencies (EC50S) for a series of primary alkanols, to resolve uncertainties about the molecular dimensions of the anaesthetic site resulting from the use of data from different laboratories. 2. For each alkanol, concentration-response relationships for loss of righting reflex (LRR) were plotted for over one hundred tadpoles, and the median effective concentrations determined. Aqueous concentrations present during potency assays were determined independently, and for alkanols with chain length greater than nonanol, correction was made for depletion from the aqueous phase. 3. The EC50S were found to decrease logarithmically with increasing number of carbon atoms in the hydrocarbon chain of the alkanol (CN), such that, on average, each additional methylene group was associated with an approximately four fold increase in potency. 4. The relationship between log EC50 and CN was best described by the quadratic equation, log EC50 = 0.022 (+/- 0.0038) CN2 + 0.76 (+/- 0.051) CN + 3.7 (+/- 0.14) (r2 = 0.9951). 5. A previously described correlation between the apparent changes in the free energy of binding of an additional methylene group both to luciferase and to the sites for LRR in tadpoles was not confirmed. 6. A cut-off in potency beyond dodecanol was established in experiments where tadpoles were maintained in supersaturated solutions of tridecanol for 20 h without demonstrable LRR. 7. These findings indicate that the soluble enzyme firefly luciferase does not adequately model the anaesthetic site. Specifically, there are discrepancies in the position of cut-off, and the apparent changes in the free energy of binding, per methylene group, of an alkanol to luciferase do not parallel that for tadpoles.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alifimoff J. K., Firestone L. L., Miller K. W. Anesthetic potencies of secondary alcohol enantiomers. Anesthesiology. 1987 Jan;66(1):55–59. doi: 10.1097/00000542-198701000-00010. [DOI] [PubMed] [Google Scholar]
- Deady J. E., Koblin D. D., Eger E. I., 2nd, Heavner J. E., D'Aoust B. Anesthetic potencies and the unitary theory of narcosis. Anesth Analg. 1981 Jun;60(6):380–384. [PubMed] [Google Scholar]
- Dodson B. A., Furmaniuk Z. W., Jr, Miller K. W. The physiological effects of hydrostatic pressure are not equivalent to those of helium pressure on Rana pipiens. J Physiol. 1985 May;362:233–244. doi: 10.1113/jphysiol.1985.sp015673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. R., Haydon D. A. Mapping of general anaesthetic target sites. Nature. 1986 Jan 2;319(6048):77–78. doi: 10.1038/319077a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Mapping of general anaesthetic target sites provides a molecular basis for cutoff effects. Nature. 1985 Jul 25;316(6026):349–351. doi: 10.1038/316349a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Partitioning of long-chain alcohols into lipid bilayers: implications for mechanisms of general anesthesia. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5116–5120. doi: 10.1073/pnas.83.14.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M. J., Miller K. W., Paton W. D., Smith E. B. Pressure reversal of anaesthesia. Nature. 1971 Jun 11;231(5302):368–371. doi: 10.1038/231368a0. [DOI] [PubMed] [Google Scholar]
- Lyon R. C., McComb J. A., Schreurs J., Goldstein D. B. A relationship between alcohol intoxication and the disordering of brain membranes by a series of short-chain alcohols. J Pharmacol Exp Ther. 1981 Sep;218(3):669–675. [PubMed] [Google Scholar]
- Pringle M. J., Brown K. B., Miller K. W. Can the lipid theories of anesthesia account for the cutoff in anesthetic potency in homologous series of alcohols? Mol Pharmacol. 1981 Jan;19(1):49–55. [PubMed] [Google Scholar]
- Richards C. D., Martin K., Gregory S., Keightley C. A., Hesketh T. R., Smith G. A., Warren G. B., Metcalfe J. C. Degenerate perturbations of protein structure as the mechanism of anaesthetic action. Nature. 1978 Dec 21;276(5690):775–779. doi: 10.1038/276775a0. [DOI] [PubMed] [Google Scholar]
- Saidman L. J., Eger E. I., 2nd, Munson E. S., Babad A. A., Muallem M. Minimum alveolar concentrations of methoxyflurane, halothane, ether and cyclopropane in man: correlation with theories of anesthesia. Anesthesiology. 1967 Nov-Dec;28(6):994–1002. doi: 10.1097/00000542-196711000-00009. [DOI] [PubMed] [Google Scholar]
- Sallee V. L. Fatty acid and alcohol partitioning with intestinal brush border and erythrocyte membranes. J Membr Biol. 1978 Oct 19;43(2-3):187–201. doi: 10.1007/BF01933478. [DOI] [PubMed] [Google Scholar]
- Vernon H. M. The changes in the reaction of growing organisms to narcotics. J Physiol. 1913 Oct 17;47(1-2):15–29. doi: 10.1113/jphysiol.1913.sp001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waud D. R. On biological assays involving quantal responses. J Pharmacol Exp Ther. 1972 Dec;183(3):577–607. [PubMed] [Google Scholar]


