Abstract
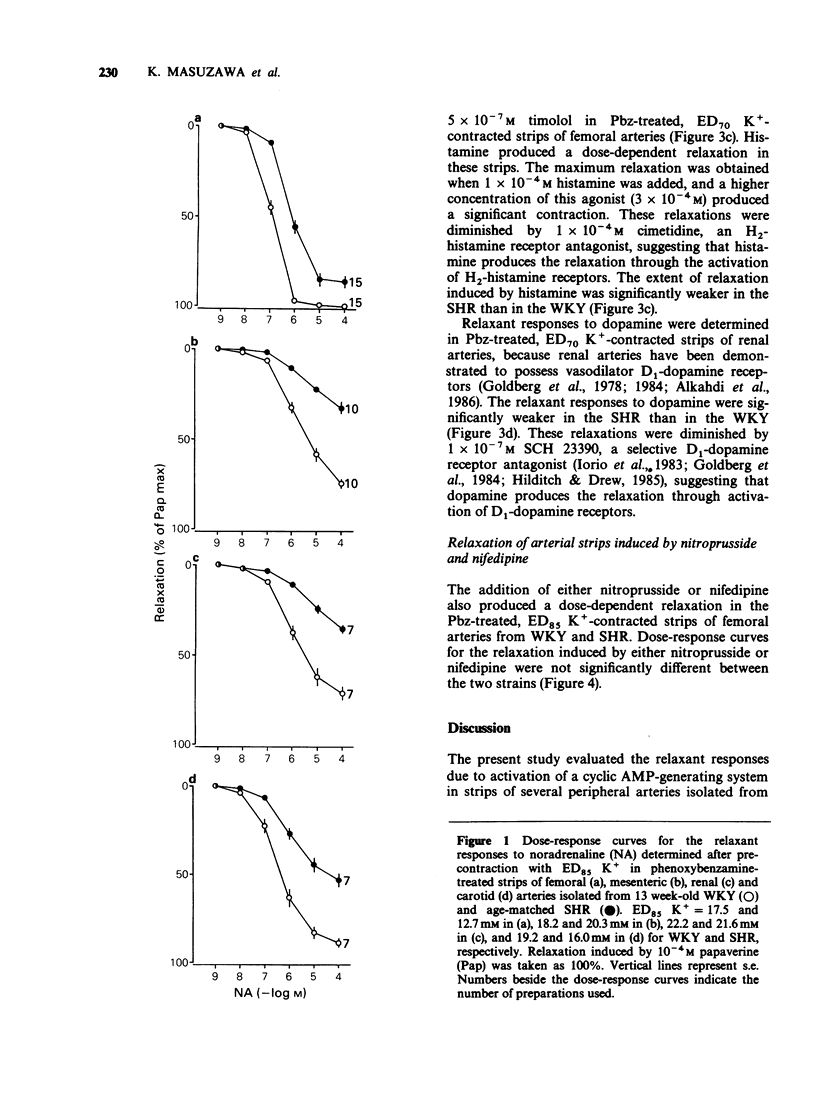
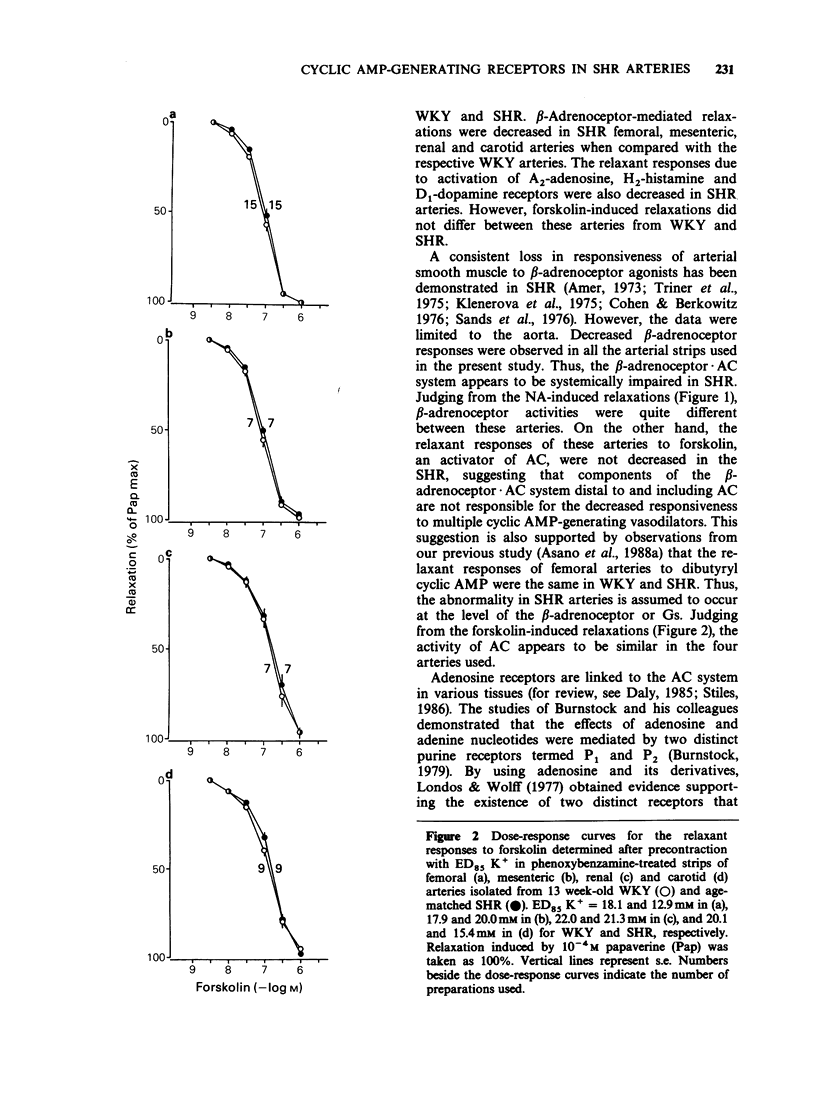
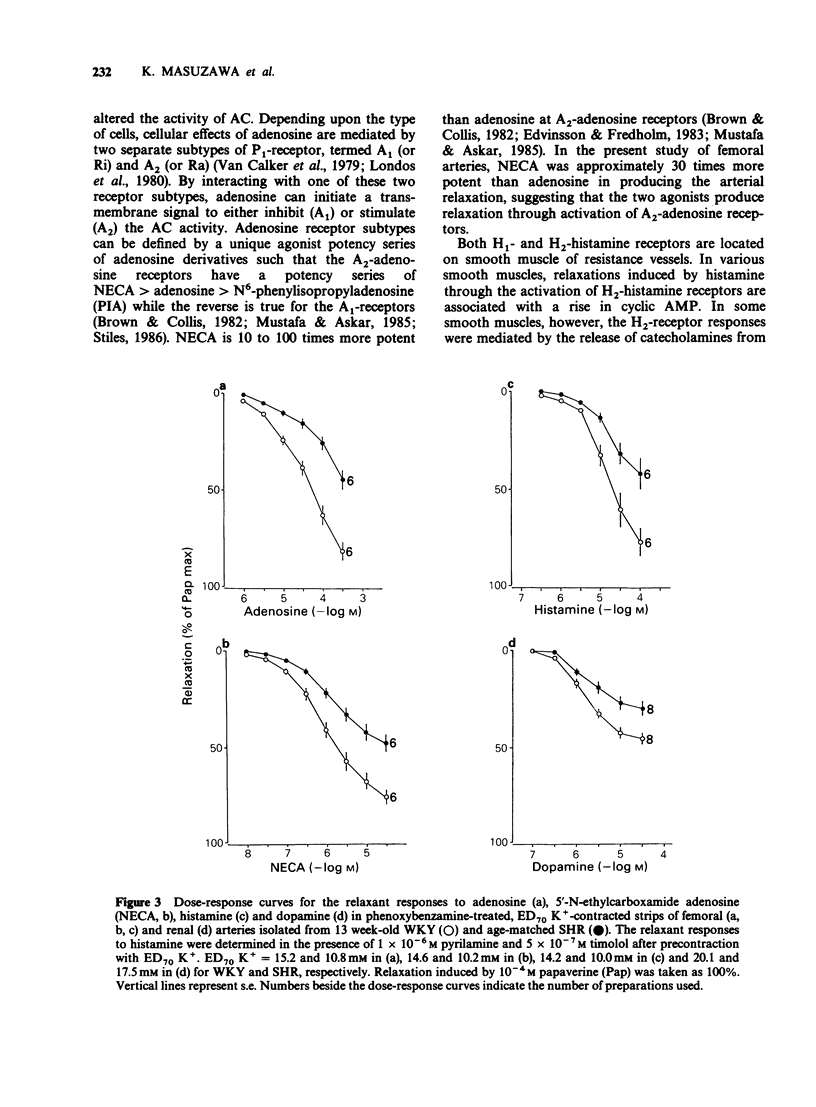
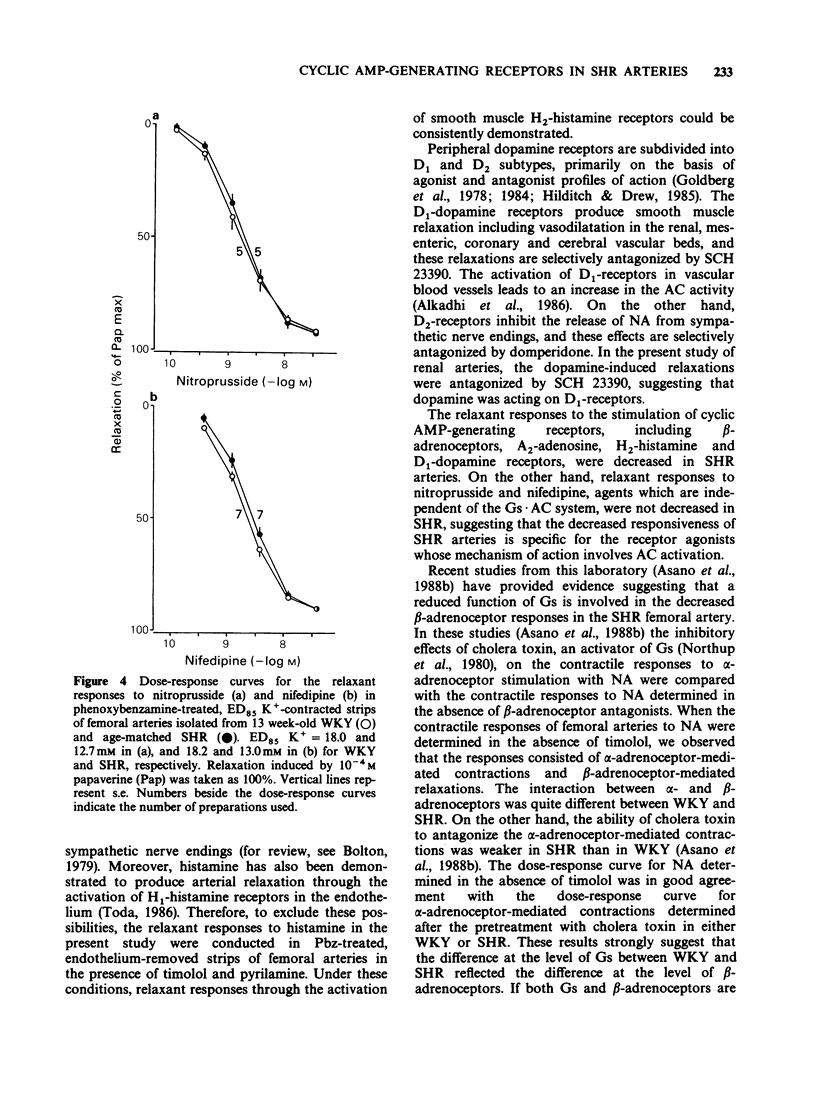
1. Arterial relaxant responses via beta-adrenoceptors are decreased in spontaneously hypertensive rats (SHR) when compared with normotensive Wistar-Kyoto rats (WKY). Recent studies from this laboratory proposed that a reduced function of stimulatory guanosine 5'-triphosphate (GTP)-binding protein (Gs) is responsible for the decreased beta-adrenoceptor responsiveness in the SHR femoral artery. Since the Gs is common to all tissues, as opposed to receptors, which are tissue specific, the reduced function of Gs should lead to resistance to multiple receptors that act by activating adenylate cyclase (AC). To test this hypothesis, relaxant responses via beta-adrenoceptors, A2-adenosine, H2-histamine and D1-dopamine receptors were compared between arterial strips from 13 week-old WKY and age-matched SHR. 2. The relaxant responses to noradrenaline (NA) via beta-adrenoceptors in femoral, mesenteric, renal and carotid arteries were significantly decreased in the SHR, when compared with the respective arteries from WKY. 3. However, under the same conditions arterial relaxant responses to forskolin, an activator of AC, were not significantly different between the WKY and SHR. 4. The relaxant responses due to activation of A2-adenosine. H2-histamine and D1-dopamine receptors were significantly decreased in the SHR arteries. 5. Nitroprusside and nifedipine, agents which are independent of the Gs.AC system, produced similar arterial relaxations in the WKY and SHR. 6. These results support the hypothesis that a reduced function of Gs in the SHR is responsible for the decreased arterial responsiveness to a variety of receptor agonists whose mechanism of action involves AC activation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkadhi K. A., Sabouni M. H., Ansari A. F., Lokhandwala M. F. Activation of DA1 receptors by dopamine or fenoldopam increases cyclic AMP levels in the renal artery but not in the superior cervical ganglion of the rat. J Pharmacol Exp Ther. 1986 Aug;238(2):547–553. [PubMed] [Google Scholar]
- Amer M. S. Cyclic adenosine monophosphate and hypertension in rats. Science. 1973 Feb 23;179(4075):807–809. doi: 10.1126/science.179.4075.807. [DOI] [PubMed] [Google Scholar]
- Asano M., Aoki K., Matsuda T. Reduced beta adrenoceptor interactions of norepinephrine enhance contraction in the femoral artery from spontaneously hypertensive rats. J Pharmacol Exp Ther. 1982 Oct;223(1):207–214. [PubMed] [Google Scholar]
- Asano M., Masuzawa K., Matsuda T. Evidence for reduced beta-adrenoceptor coupling to adenylate cyclase in femoral arteries from spontaneously hypertensive rats. Br J Pharmacol. 1988 May;94(1):73–86. doi: 10.1111/j.1476-5381.1988.tb11501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano M., Masuzawa K., Matsuda T. Role of stimulatory GTP-binding protein (Gs) in reduced beta-adrenoceptor coupling in the femoral artery of spontaneously hypertensive rats. Br J Pharmacol. 1988 Sep;95(1):241–251. doi: 10.1111/j.1476-5381.1988.tb16570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Collis M. G. Evidence for an A2/Ra adenosine receptor in the guinea-pig trachea. Br J Pharmacol. 1982 Jul;76(3):381–387. doi: 10.1111/j.1476-5381.1982.tb09231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. L., Berkowitz B. A. Decreased vascular relaxation in hypertension. J Pharmacol Exp Ther. 1976 Feb;196(2):396–406. [PubMed] [Google Scholar]
- Daly J. W. Adenosine receptors. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:29–46. [PubMed] [Google Scholar]
- Edvinsson L., Fredholm B. B. Characterization of adenosine receptors in isolated cerebral arteries of cat. Br J Pharmacol. 1983 Dec;80(4):631–637. doi: 10.1111/j.1476-5381.1983.tb10052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goldberg L. I., Volkman P. H., Kohli J. D. A comparison of the vascular dopamine receptor with other dopamine receptors. Annu Rev Pharmacol Toxicol. 1978;18:57–79. doi: 10.1146/annurev.pa.18.040178.000421. [DOI] [PubMed] [Google Scholar]
- Iorio L. C., Barnett A., Leitz F. H., Houser V. P., Korduba C. A. SCH 23390, a potential benzazepine antipsychotic with unique interactions on dopaminergic systems. J Pharmacol Exp Ther. 1983 Aug;226(2):462–468. [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos C., Wolff J. Two distinct adenosine-sensitive sites on adenylate cyclase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5482–5486. doi: 10.1073/pnas.74.12.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa S. J., Askar A. O. Evidence suggesting an Ra-type adenosine receptor in bovine coronary arteries. J Pharmacol Exp Ther. 1985 Jan;232(1):49–56. [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Smigel M. D., Schleifer L. S., Ross E. M., Gilman A. G. Purification of the regulatory component of adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6516–6520. doi: 10.1073/pnas.77.11.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands H., Sinclair D., Mascali J. Cyclic AMP and protein kinase in the spontaneously hypertensive rat aorta and tissue-cultured aortic smooth muscle cells. Blood Vessels. 1976;13(6):361–373. doi: 10.1159/000158106. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Toda N. Mechanisms of histamine-induced relaxation in isolated monkey and dog coronary arteries. J Pharmacol Exp Ther. 1986 Nov;239(2):529–535. [PubMed] [Google Scholar]
- Triner L., Vulliemoz Y., Verosky M., Manger W. M. Cyclic adenosine monophosphate and vascular reactivity in spontaneously hypertensive rats. Biochem Pharmacol. 1975 Mar 15;24(6):743–745. doi: 10.1016/0006-2952(75)90253-1. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


