Abstract
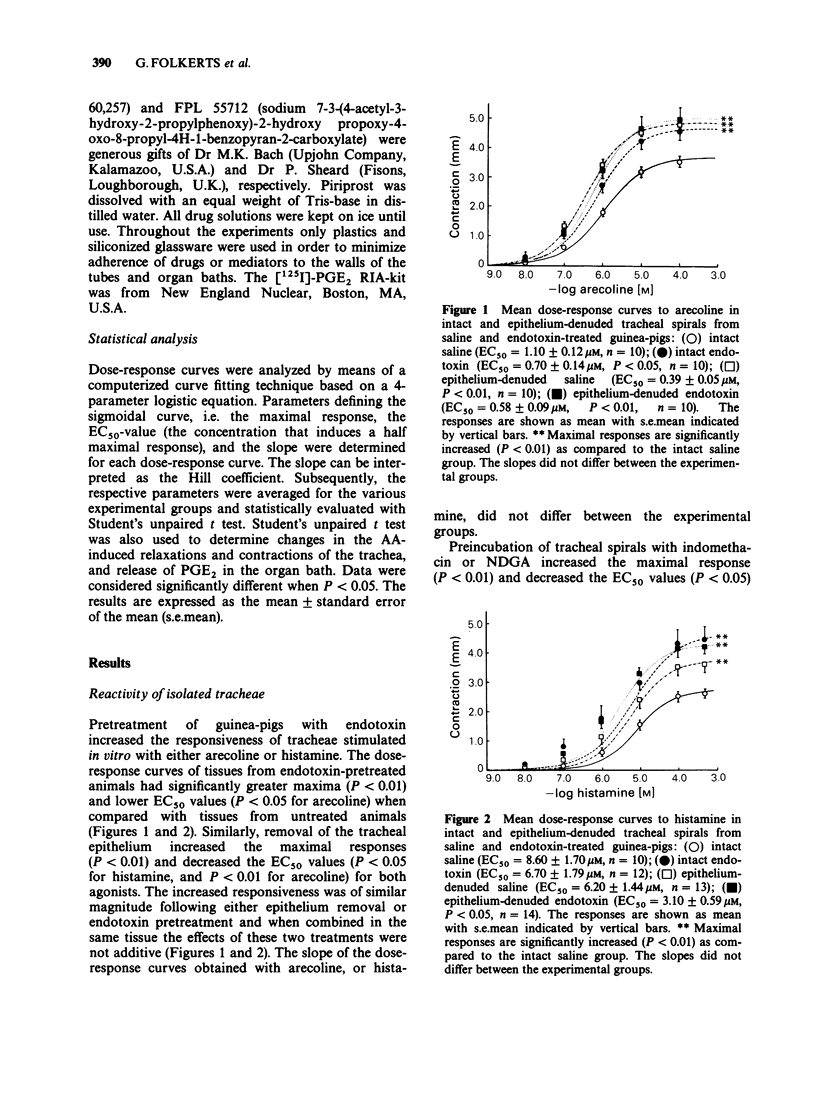
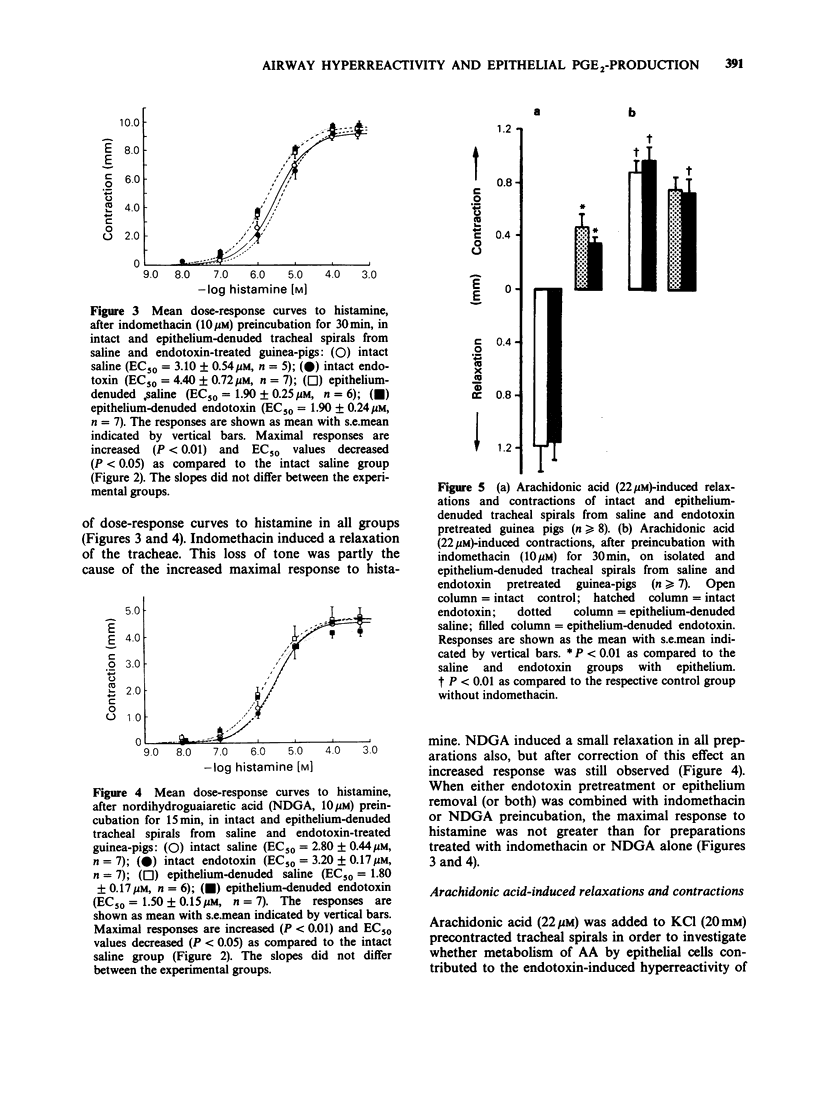
1. Pretreatment of guinea-pigs with endotoxin (1 mg kg-1 b.w., i.p., 4 days before the experiments) results in respiratory airway hyperreactivity in vitro. Dose-response curves with either arecoline or histamine on isolated tracheae from these animals display increased maximal contractions, and decreased EC50 values. 2. Tracheae denuded of epithelium respond with a similar hyperreactivity to histamine as observed in preparations from endotoxin pretreated animals. Removal of the epithelial layer of tracheae from endotoxin pretreated guinea-pigs did not additionally affect the histamine dose-response curve. 3. The cyclo-oxygenase inhibitor indomethacin (10 microM) induces histamine hyperreactivity which is equal in intact and epithelium-denuded tracheae from saline or endotoxin pretreated guinea-pigs. Similar results are obtained with the combined lipoxygenase/cyclo-oxygenase inhibitor nordihydroguaiaretic acid (10 microM). 4. Histamine (0.1 mM) induces an increase in prostaglandin E2 (PGE2) formation by the tracheal spiral in vitro, which is reduced by 34% by endotoxin pretreatment, and by about 60% following epithelium removal irrespective of endotoxin pretreatment. 5. Arachidonic acid (AA, 22 microM) stimulation of the guinea-pig trachea in vitro induces a relaxation, and an increase in PGE2 production. In preparations lacking the epithelium, AA induces a contraction which coincides with a 60% reduced increase in PGE2 formation. These effects are not altered by endotoxin pretreatment. 6. It is concluded that the endotoxin-induced respiratory airway hyperreactivity may be caused by a disturbed ability of epithelial cells to synthesize PGE2. The decreased formation of this prostaglandin is rather the consequence of a diminished liberation of AA from the phospholipid stores than a dysfunction of the cyclo-oxygenase enzyme.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa H., Miyazaki N., Shigematsu N., Tomooka M. A possible role of airway epithelium in modulating hyperresponsiveness. Br J Pharmacol. 1988 Jan;93(1):139–145. doi: 10.1111/j.1476-5381.1988.tb11414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. H., Krzanowski J. J., Polson J. B., Szentivanyi A. The effect of prostaglandin E2 on histamine-stimulated calcium mobilization as a possible explanation for histamine tachyphylaxis in canine tracheal smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1983 Feb;322(1):72–77. doi: 10.1007/BF00649355. [DOI] [PubMed] [Google Scholar]
- Barnes P. J., Cuss F. M., Palmer J. B. The effect of airway epithelium on smooth muscle contractility in bovine trachea. Br J Pharmacol. 1985 Nov;86(3):685–691. doi: 10.1111/j.1476-5381.1985.tb08946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell G. J., Flower R. J., Nijkamp F. P., Vane J. R. Phospholipase A2 activity of guinea-pig isolated perfused lungs: stimulation, and inhibition by anti-inflammatory steroids. Br J Pharmacol. 1978 Jan;62(1):79–89. doi: 10.1111/j.1476-5381.1978.tb07009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein G., Labat C., Brunelleschi S., Benveniste J., Marsac J., Brink C. Evidence that the histamine sensitivity and responsiveness of guinea-pig isolated trachea are modulated by epithelial prostaglandin E2 production. Br J Pharmacol. 1988 Sep;95(1):300–308. doi: 10.1111/j.1476-5381.1988.tb16577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelleschi S., Haye-Legrand I., Labat C., Norel X., Benveniste J., Brink C. Platelet-activating factor-acether-induced relaxation of guinea pig airway muscle: role of prostaglandin E2 and the epithelium. J Pharmacol Exp Ther. 1987 Oct;243(1):356–363. [PubMed] [Google Scholar]
- Butler G. B., Adler K. B., Evans J. N., Morgan D. W., Szarek J. L. Modulation of rabbit airway smooth muscle responsiveness by respiratory epithelium. Involvement of an inhibitory metabolite of arachidonic acid. Am Rev Respir Dis. 1987 May;135(5):1099–1104. doi: 10.1164/arrd.1987.135.5.1099. [DOI] [PubMed] [Google Scholar]
- Engels F., Folkerts G., van Heuven-Nolsen D., Nijkamp F. P. Haemophilus influenzae-induced decreases in lung beta-adrenoceptor function and number coincide with decreases in spleen noradrenaline. Naunyn Schmiedebergs Arch Pharmacol. 1987 Sep;336(3):274–279. doi: 10.1007/BF00172678. [DOI] [PubMed] [Google Scholar]
- Farmer S. G., Hay D. W., Raeburn D., Fedan J. S. Relaxation of guinea-pig tracheal smooth muscle to arachidonate is converted to contraction following epithelium removal. Br J Pharmacol. 1987 Sep;92(1):231–236. doi: 10.1111/j.1476-5381.1987.tb11316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavahan N. A., Aarhus L. L., Rimele T. J., Vanhoutte P. M. Respiratory epithelium inhibits bronchial smooth muscle tone. J Appl Physiol (1985) 1985 Mar;58(3):834–838. doi: 10.1152/jappl.1985.58.3.834. [DOI] [PubMed] [Google Scholar]
- Folkerts G., Henricks P. A., Slootweg P. J., Nijkamp F. P. Endotoxin-induced inflammation and injury of the guinea pig respiratory airways cause bronchial hyporeactivity. Am Rev Respir Dis. 1988 Jun;137(6):1441–1448. doi: 10.1164/ajrccm/137.6.1441. [DOI] [PubMed] [Google Scholar]
- Folkerts G., Nijkamp F. P. Haemophilus influenzae induces a potentiated increase in guinea-pig pulmonary resistance to histamine. Eur J Pharmacol. 1985 Dec 10;119(1-2):117–120. doi: 10.1016/0014-2999(85)90329-2. [DOI] [PubMed] [Google Scholar]
- Folkerts G., Nijkamp F. P., van Oosterhout A. J. Induction in guinea-pigs of airway hyperreactivity and decreased lung beta-adrenoceptor number by 15-hydroperoxy-arachidonic acid. Br J Pharmacol. 1983 Dec;80(4):597–599. doi: 10.1111/j.1476-5381.1983.tb10047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinska L., Panczenko B., Gryglewski R. J. Generation of prostaglandin E-like material by the guinea-pig trachea contracted by histamine. J Pharm Pharmacol. 1975 Feb;27(2):88–91. doi: 10.1111/j.2042-7158.1975.tb09414.x. [DOI] [PubMed] [Google Scholar]
- Ku E. C., Lee W., Kothari H. V., Kimble E. F., Liauw L., Tjan J. The effects of diclofenac sodium on arachidonic acid metabolism. Semin Arthritis Rheum. 1985 Nov;15(2 Suppl 1):36–41. doi: 10.1016/s0049-0172(85)80008-1. [DOI] [PubMed] [Google Scholar]
- Laitinen L. A., Heino M., Laitinen A., Kava T., Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985 Apr;131(4):599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- Mathé A. A., Hedqvist P., Strandberg K., Leslie C. A. Aspects of prostaglandin function in the lung (second of two parts). N Engl J Med. 1977 Apr 21;296(16):910–914. doi: 10.1056/NEJM197704212961605. [DOI] [PubMed] [Google Scholar]
- Nijkamp F. P., Folkerts G. Reversal of arachidonic acid-induced guinea-pig tracheal relaxation into contraction after epithelium removal. Eur J Pharmacol. 1986 Nov 19;131(2-3):315–316. doi: 10.1016/0014-2999(86)90591-1. [DOI] [PubMed] [Google Scholar]
- Orehek J., Douglas J. S., Bouhuys A. Contractile responses of the guinea-pig trachea in vitro: modification by prostaglandin synthesis-inhibiting drugs. J Pharmacol Exp Ther. 1975 Sep;194(3):554–564. [PubMed] [Google Scholar]
- Orehek J., Douglas J. S., Lewis A. J., Bouhuys A. Prostaglandin regulation of airway smooth muscle tone. Nat New Biol. 1973 Sep 19;245(142):84–85. doi: 10.1038/newbio245084a0. [DOI] [PubMed] [Google Scholar]
- Schreurs A. J., Verhoef J., Nijkamp F. P. Bacterial cell wall components decrease the number of guinea-pig lung beta-adrenoceptors. Eur J Pharmacol. 1983 Jan 28;87(1):127–132. doi: 10.1016/0014-2999(83)90058-4. [DOI] [PubMed] [Google Scholar]
- Steel L., Platshon L., Kaliner M. Prostaglandin generation by human and guinea pig lung tissue: comparison of parenchymal and airway responses. J Allergy Clin Immunol. 1979 Oct;64(4):287–293. doi: 10.1016/0091-6749(79)90146-5. [DOI] [PubMed] [Google Scholar]
- Van Oosterhout A. J., Folkerts G., Ten Have G. A., Nijkamp F. P. Involvement of the spleen in the endotoxin-induced deterioration of the respiratory airway and lymphocyte beta-adrenergic systems of the guinea pig. Eur J Pharmacol. 1988 Mar 15;147(3):421–429. doi: 10.1016/0014-2999(88)90177-x. [DOI] [PubMed] [Google Scholar]
- van Heuven-Nolsen D., Folkerts G., de Wildt D. J., Nijkamp F. P. The influence of Bordetella pertussis and its constituents on the beta-adrenergic receptor in the guinea pig respiratory system. Life Sci. 1986 Feb 24;38(8):677–685. doi: 10.1016/0024-3205(86)90581-3. [DOI] [PubMed] [Google Scholar]


