Abstract
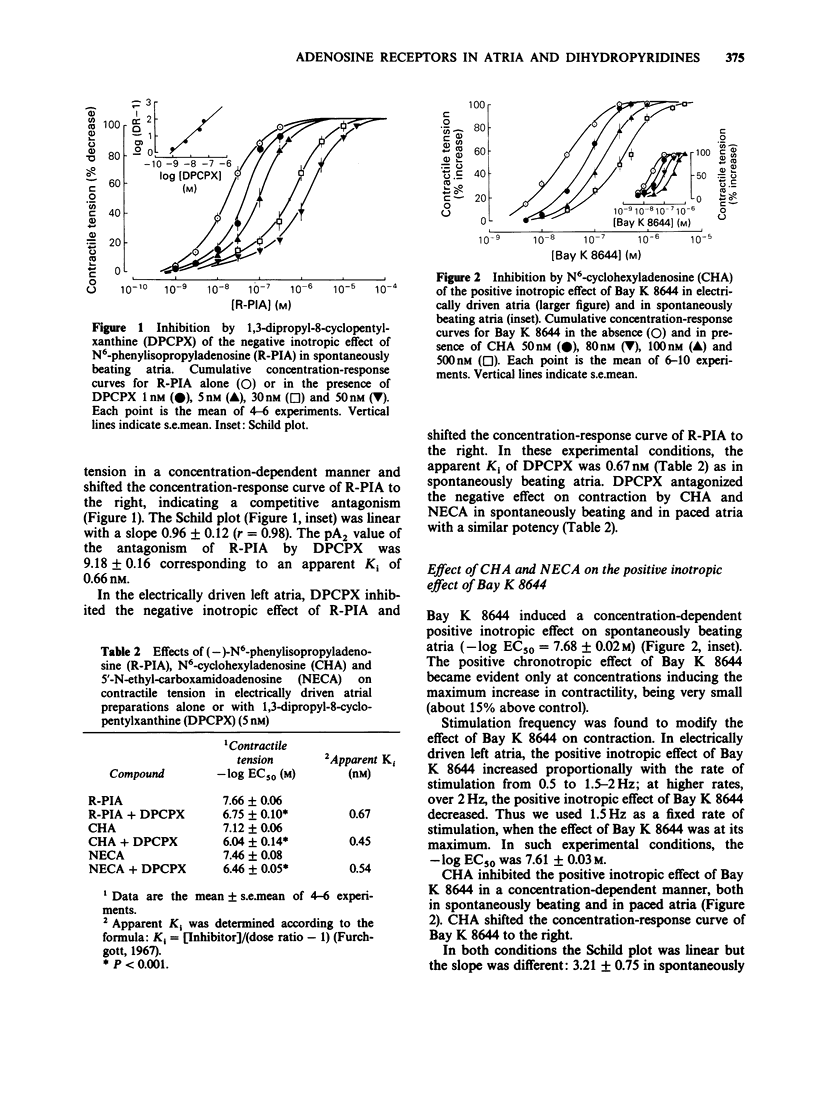
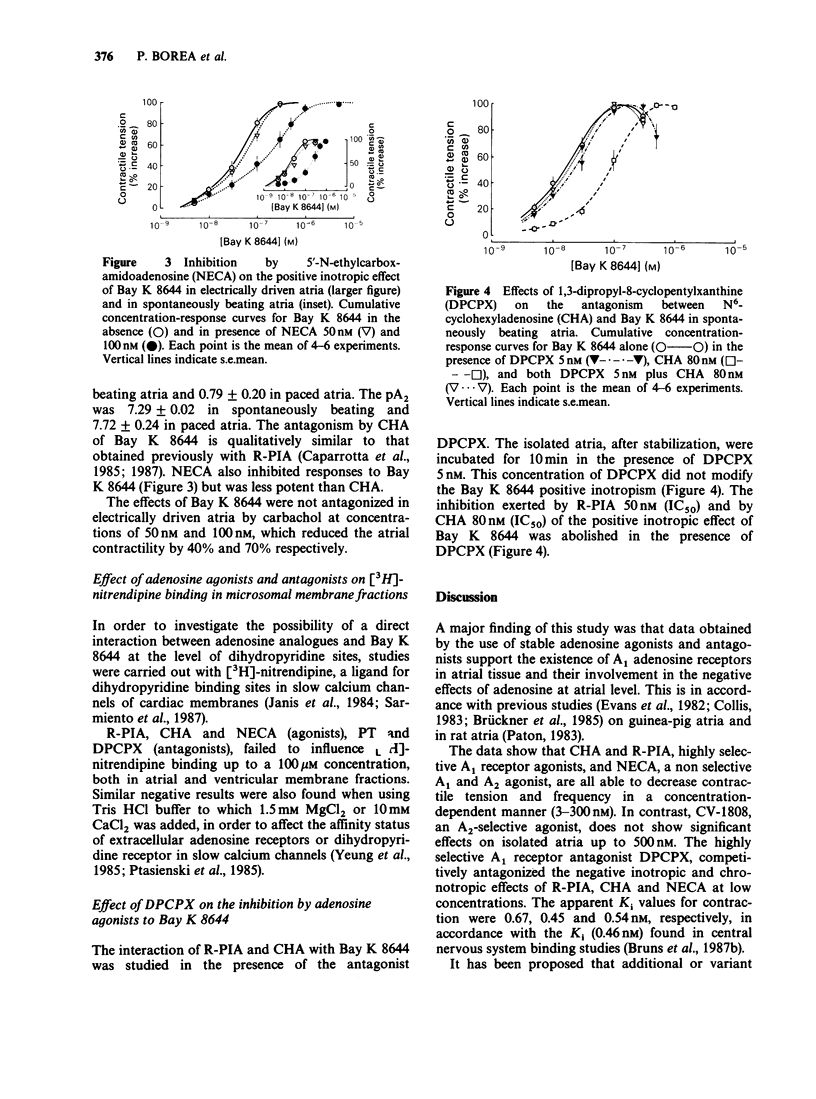
1. (-)-N6-phenylisopropyladenosine (R-PIA) and N6-cyclohexyladenosine (CHA), highly selective agonists at A1-adenosine receptors, 5'-N-ethyl-carboxamidoadenosine (NECA), a non-selective agonist at A1 and A2 receptors, and 2-phenylaminoadenosine (CV-1808), a selective A2 agonist, were compared in spontaneously beating and electrically driven atria. R-PIA, CHA and NECA inhibited contraction in both preparations. CV-1808 was not effective up to 500 nM. 2. 1,3-Dipropyl-8-cyclopentylxanthine (DPCPX), a new selective A1 receptor antagonist, competitively inhibited the effects of the adenosine agonists, at low concentrations (IC50 less than 1 nM). 3. CHA and NECA were able to inhibit the positive inotropic effect of Bay K 8644 both in spontaneously beating and in electrically driven atria. 4. R-PIA, CHA and NECA (agonists), 8-phenyltheophylline (PT) and DPCPX (antagonists), failed to influence [3H]-nitrendipine binding on microsomal membranes from guinea-pig atria and ventricles in a range of concentrations from 1 nM to 100 microM. 5. The data support the existence of A1 receptors in atrial tissue. No evidence for a direct interaction between adenosine analogues and Bay K 8644 was found at the level of slow calcium channels. Adenosine analogues appear to antagonize the effects of Bay K 8644 indirectly by activation of A1 receptors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger G. T., Gengo P. J., Luchowski E. M., Siegel H., Triggle D. J., Janis R. A. High affinity binding of a calcium channel antagonist to smooth and cardiac muscle. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1604–1609. doi: 10.1016/0006-291x(82)91436-x. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Fergus J. H., Badger E. W., Bristol J. A., Santay L. A., Hartman J. D., Hays S. J., Huang C. C. Binding of the A1-selective adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine to rat brain membranes. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jan;335(1):59–63. doi: 10.1007/BF00165037. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Lu G. H., Pugsley T. A. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol. 1986 Apr;29(4):331–346. [PubMed] [Google Scholar]
- Brückner R., Fenner A., Meyer W., Nobis T. M., Schmitz W., Scholz H. Cardiac effects of adenosine and adenosine analogs in guinea-pig atrial and ventricular preparations: evidence against a role of cyclic AMP and cyclic GMP. J Pharmacol Exp Ther. 1985 Sep;234(3):766–774. [PubMed] [Google Scholar]
- Böhm M., Brückner R., Neumann J., Schmitz W., Scholz H., Starbatty J. Role of guanine nucleotide-binding protein in the regulation by adenosine of cardiac potassium conductance and force of contraction. Evaluation with pertussis toxin. Naunyn Schmiedebergs Arch Pharmacol. 1986 Apr;332(4):403–405. doi: 10.1007/BF00500095. [DOI] [PubMed] [Google Scholar]
- Caparrotta L., Fassina G., Froldi G., Poja R. Antagonism between (-)-N6-phenylisopropyladenosine and the calcium channel facilitator Bay K 8644, on guinea-pig isolated atria. Br J Pharmacol. 1987 Jan;90(1):23–30. doi: 10.1111/j.1476-5381.1987.tb16821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis M. G. Evidence for an A1-adenosine receptor in the guinea-pig atrium. Br J Pharmacol. 1983 Jan;78(1):207–212. doi: 10.1111/j.1476-5381.1983.tb09381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. B., Schenden J. A., Bristol J. A. Adenosine receptors mediating cardiac depression. Life Sci. 1982 Nov 29;31(22):2425–2432. doi: 10.1016/0024-3205(82)90746-9. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. The pharmacological differentiation of adrenergic receptors. Ann N Y Acad Sci. 1967 Feb 10;139(3):553–570. doi: 10.1111/j.1749-6632.1967.tb41229.x. [DOI] [PubMed] [Google Scholar]
- Haleen S. J., Steffen R. P., Hamilton H. W. PD 116,948, a highly selective A1 adenosine receptor antagonist. Life Sci. 1987 Feb 9;40(6):555–561. doi: 10.1016/0024-3205(87)90369-9. [DOI] [PubMed] [Google Scholar]
- Jacobson K. A., Kirk K. L., Padgett W. L., Daly J. W. Functionalized congeners of 1,3-dialkylxanthines: preparation of analogues with high affinity for adenosine receptors. J Med Chem. 1985 Sep;28(9):1334–1340. doi: 10.1021/jm00147a038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis R. A., Rampe D., Sarmiento J. G., Triggle D. J. Specific binding of a calcium channel activator, [3H]BAY k 8644, to membranes from cardiac muscle and brain. Biochem Biophys Res Commun. 1984 May 31;121(1):317–323. doi: 10.1016/0006-291x(84)90725-3. [DOI] [PubMed] [Google Scholar]
- Janis R. A., Sarmiento J. G., Maurer S. C., Bolger G. T., Triggle D. J. Characteristics of the binding of [3H]nitrendipine to rabbit ventricular membranes: modification by other Ca++ channel antagonists and by the Ca++ channel agonist Bay K 8644. J Pharmacol Exp Ther. 1984 Oct;231(1):8–15. [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986 Sep;407(3):264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAMURA M., STONE R. L., KRUBSACK J. E. SURVIVAL OF SHIGELLA IN SEA WATER. Nature. 1964 Jul 11;203:213–214. doi: 10.1038/203213a0. [DOI] [PubMed] [Google Scholar]
- Ptasienski J., McMahon K. K., Hosey M. M. High and low affinity states of the dihydropyridine and phenylalkylamine receptors on the cardiac calcium channel and their interconversion by divalent cations. Biochem Biophys Res Commun. 1985 Jun 28;129(3):910–917. doi: 10.1016/0006-291x(85)91978-3. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. A., Sebastião A. M. Adenosine receptors and calcium: basis for proposing a third (A3) adenosine receptor. Prog Neurobiol. 1986;26(3):179–209. doi: 10.1016/0301-0082(86)90015-8. [DOI] [PubMed] [Google Scholar]
- Sarmiento J. G., Shrikhande A. V., Janis R. A., Triggle D. J. [3H]BAY K 8644, a 1,4-dihydropyridine Ca++ channel activator: characteristics of binding to high and low affinity sites in cardiac membranes. J Pharmacol Exp Ther. 1987 Apr;241(1):140–146. [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983 Jun 9;303(5917):535–537. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- Williams M. Purine receptors in mammalian tissues: pharmacology and functional significance. Annu Rev Pharmacol Toxicol. 1987;27:315–345. doi: 10.1146/annurev.pa.27.040187.001531. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


