Abstract
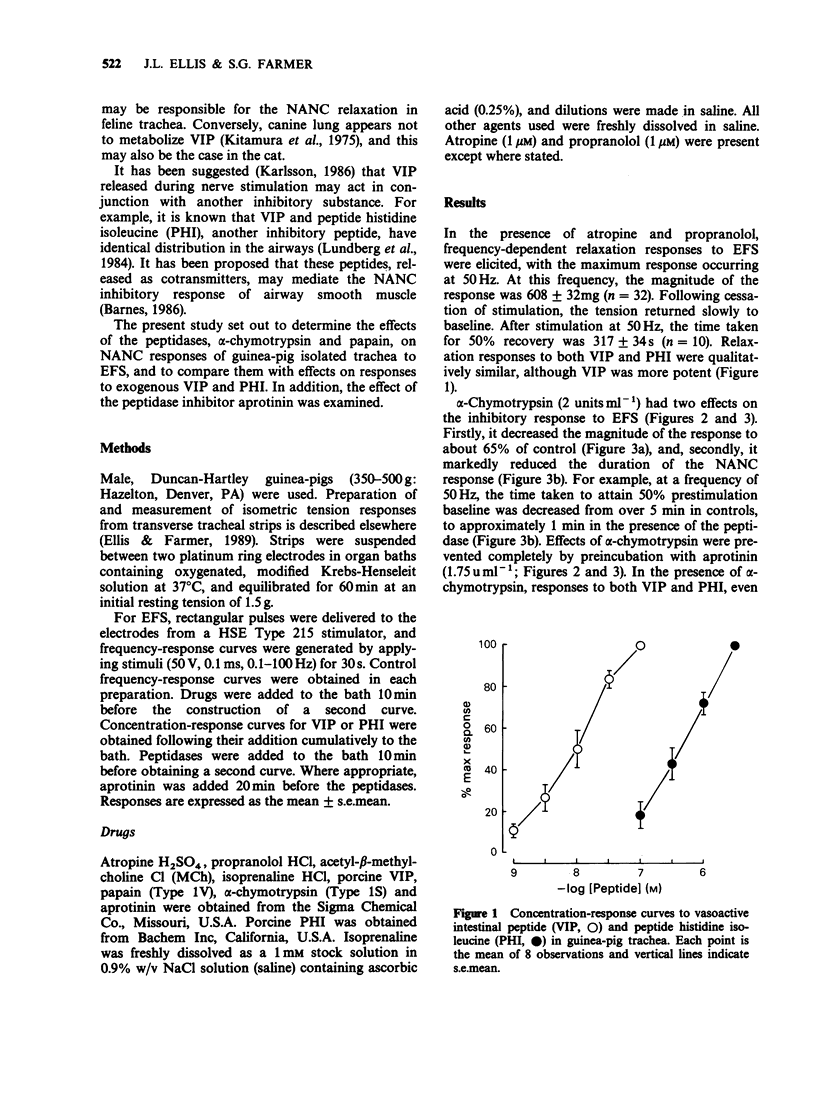
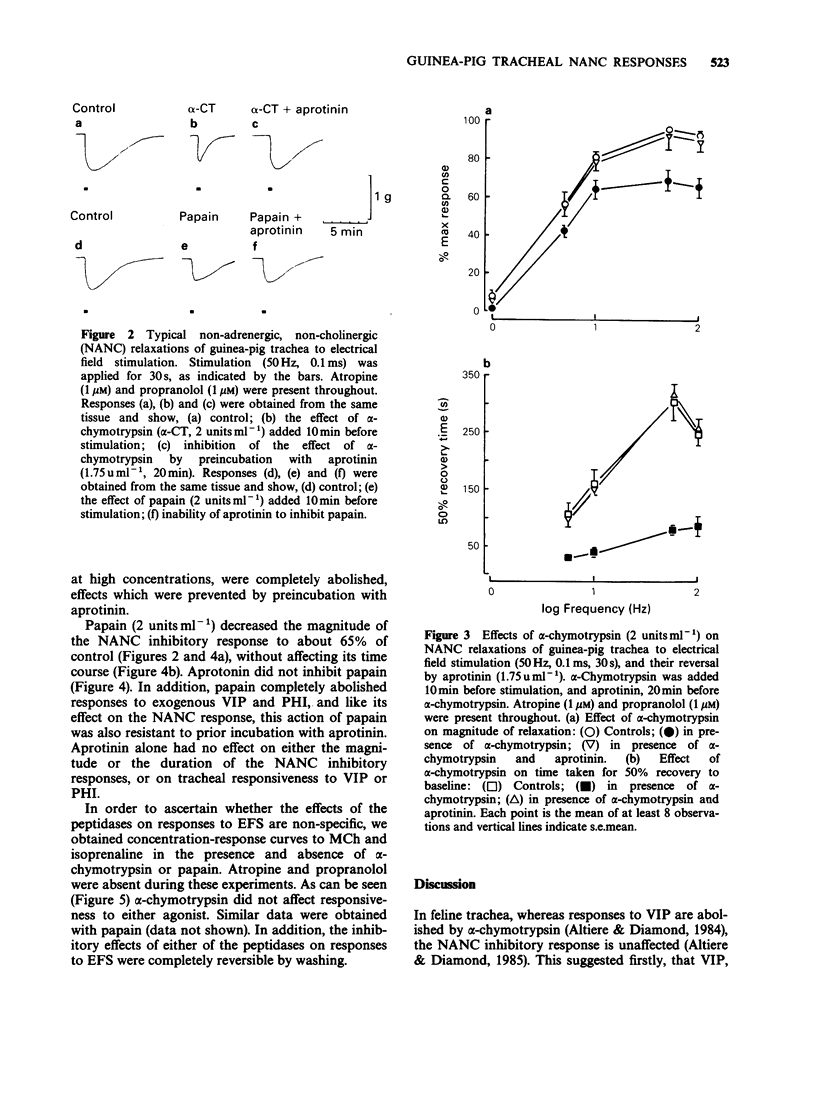
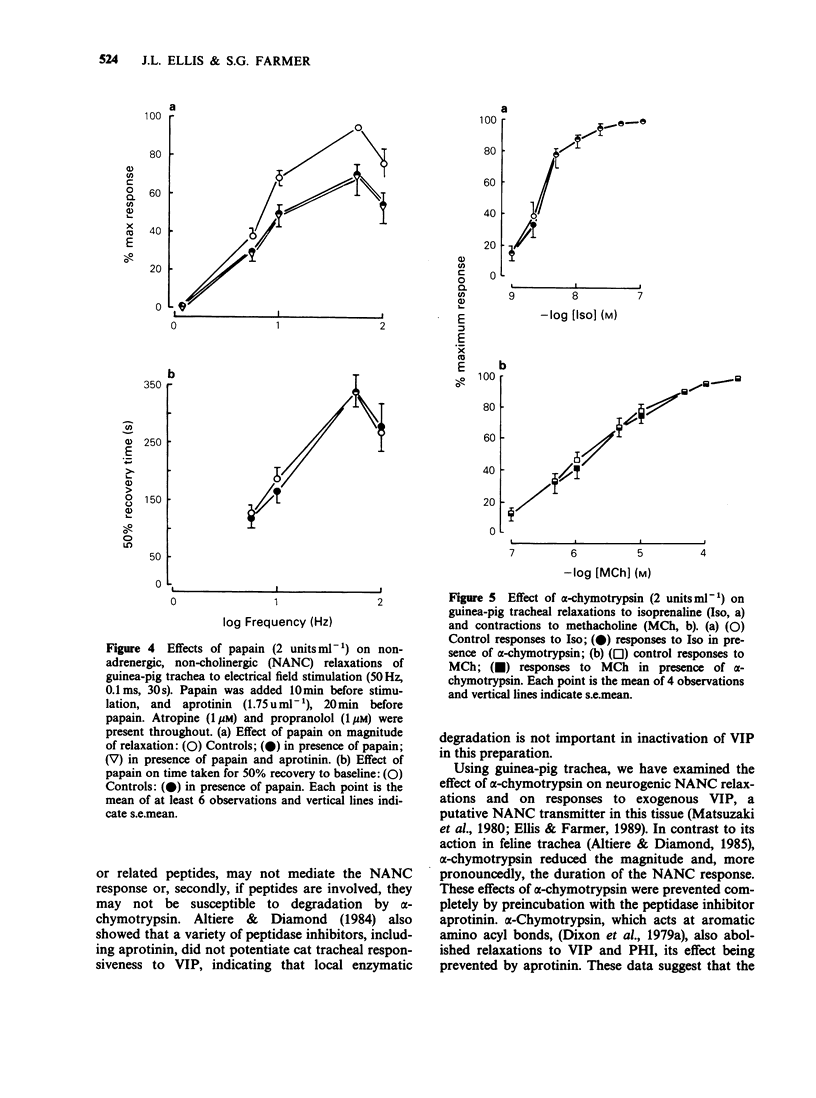
1. The effects of peptidase enzymes on non-adrenergic, non-cholinergic (NANC) inhibitory responses of guinea-pig trachea to electrical field stimulation (EFS), and on relaxations induced by vasoactive intestinal peptide (VIP) and peptide histidine isoleucine (PHI) have been examined. 2. alpha-Chymotrypsin reduced both the magnitude and, particularly, the duration of the inhibitory response to EFS, whereas papain reduced only the magnitude. Aprotinin, a peptidase inhibitor prevented the effects of alpha-chymotrypsin but was without effect on papain. 3. alpha-Chymotrypsin and papain both abolished relaxant responses to exogenous VIP and PHI. The action of alpha-chymotrypsin was prevented by aprotinin, whereas that of papain was not affected. 4. The peptidases were without effect on concentration-response curves to methacholine or to isoprenaline. It was also observed that, in the absence of the peptidases, aprotinin had no effect on inhibitory responses either to EFS or to exogenous VIP and PHI. 5. It is suggested that neuropeptides, possibly VIP and PHI, released during EFS of guinea-pig trachea, partly mediate NANC relaxations, and that their action may be inhibited by peptidases. However, the lack of effect of aprotinin alone, on responses to EFS, suggests that, if endogenous peptidases are important in terminating the action of neuropeptides, they are resistant to the effect of this particular peptidase inhibitor. It is further suggested that neurogenic relaxation of guinea-pig trachea is also partly mediated by a substance, possibly non-peptide, other than VIP or PHI.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altiere R. J., Diamond L. Effect of alpha-chymotrypsin on the nonadrenergic noncholinergic inhibitory system in cat airways. Eur J Pharmacol. 1985 Aug 7;114(1):75–78. doi: 10.1016/0014-2999(85)90523-0. [DOI] [PubMed] [Google Scholar]
- Altiere R. J., Diamond L. Relaxation of cat tracheobronchial and pulmonary arterial smooth muscle by vasoactive intestinal peptide: lack of influence by peptidase inhibitors. Br J Pharmacol. 1984 Jun;82(2):321–328. doi: 10.1111/j.1476-5381.1984.tb10766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel F., Go V. L., Schmalz P. F., Szurszewski J. H. Vasoactive intestinal polypeptide: a putative transmitter in the canine gastric muscularis mucosa. J Physiol. 1983 Aug;341:641–654. doi: 10.1113/jphysiol.1983.sp014830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. J. Neural control of human airways in health and disease. Am Rev Respir Dis. 1986 Dec;134(6):1289–1314. doi: 10.1164/arrd.1986.134.5.1289. [DOI] [PubMed] [Google Scholar]
- Bodanszky M., Klausner Y. S., Said S. I. Biological activities of synthetic peptides corresponding to fragments of and to the entire sequence of the vasoactive intestinal peptide. Proc Natl Acad Sci U S A. 1973 Feb;70(2):382–384. doi: 10.1073/pnas.70.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnett N. W. The role of neuropeptides in regulating airway function. Postsecretory metabolism of peptides. Am Rev Respir Dis. 1987 Dec;136(6 Pt 2):S27–S34. doi: 10.1164/ajrccm/136.6_Pt_2.S27. [DOI] [PubMed] [Google Scholar]
- Burnstock G. The non-adrenergic non-cholinergic nervous system. Arch Int Pharmacodyn Ther. 1986 Apr;280(2 Suppl):1–15. [PubMed] [Google Scholar]
- Caughey G. H., Leidig F., Viro N. F., Nadel J. A. Substance P and vasoactive intestinal peptide degradation by mast cell tryptase and chymase. J Pharmacol Exp Ther. 1988 Jan;244(1):133–137. [PubMed] [Google Scholar]
- De Beurme F. A., Lefebvre R. A. Influence of alpha-chymotrypsin and trypsin on the non-adrenergic non-cholinergic relaxation in the rat gastric fundus. Br J Pharmacol. 1987 May;91(1):171–177. doi: 10.1111/j.1476-5381.1987.tb08996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. L., Farmer S. G. Effects of peptidases on non-adrenergic, non-cholinergic inhibitory responses of tracheal smooth muscle: a comparison with effects on VIP- and PHI-induced relaxation. Br J Pharmacol. 1989 Mar;96(3):521–526. doi: 10.1111/j.1476-5381.1989.tb11848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Takeda K. Non-adrenergic inhibitory nerves and putative transmitters in the smooth muscle of cat trachea. J Physiol. 1982 Sep;330:497–511. doi: 10.1113/jphysiol.1982.sp014355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J. A. In vivo and in vitro studies of the non-adrenergic non-cholinergic nervous system of the guinea-pig airways. Arch Int Pharmacodyn Ther. 1986 Apr;280(2 Suppl):191–207. [PubMed] [Google Scholar]
- Kitamura S., Yoshida T., Said S. I. Vasoactive intestinal polypoptide: inactivation in liver and potentiation in lung of anesthetized dogs (384699). Proc Soc Exp Biol Med. 1975 Jan;148(1):25–29. doi: 10.3181/00379727-148-38469. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Fahrenkrug J., Hökfelt T., Martling C. R., Larsson O., Tatemoto K., Anggård A. Co-existence of peptide HI (PHI) and VIP in nerves regulating blood flow and bronchial smooth muscle tone in various mammals including man. Peptides. 1984 May-Jun;5(3):593–606. doi: 10.1016/0196-9781(84)90090-1. [DOI] [PubMed] [Google Scholar]
- Matsuzaki Y., Hamasaki Y., Said S. I. Vasoactive intestinal peptide: a possible transmitter of nonadrenergic relaxation of guinea pig airways. Science. 1980 Dec 12;210(4475):1252–1253. doi: 10.1126/science.6254154. [DOI] [PubMed] [Google Scholar]
- Staun-Olsen P., Ottesen B., Gammeltoft S., Fahrenkrug J. VIP binding sites on synaptosomes from rat cerebral cortex: structure-binding relationship. Peptides. 1986;7 (Suppl 1):181–186. doi: 10.1016/0196-9781(86)90183-x. [DOI] [PubMed] [Google Scholar]
- Zyznar E. S. A rationale for the application of trasylol as a protease inhibitor in radioimmunoassay. Life Sci. 1981 Apr 27;28(17):1861–1866. doi: 10.1016/0024-3205(81)90291-5. [DOI] [PubMed] [Google Scholar]


