Abstract
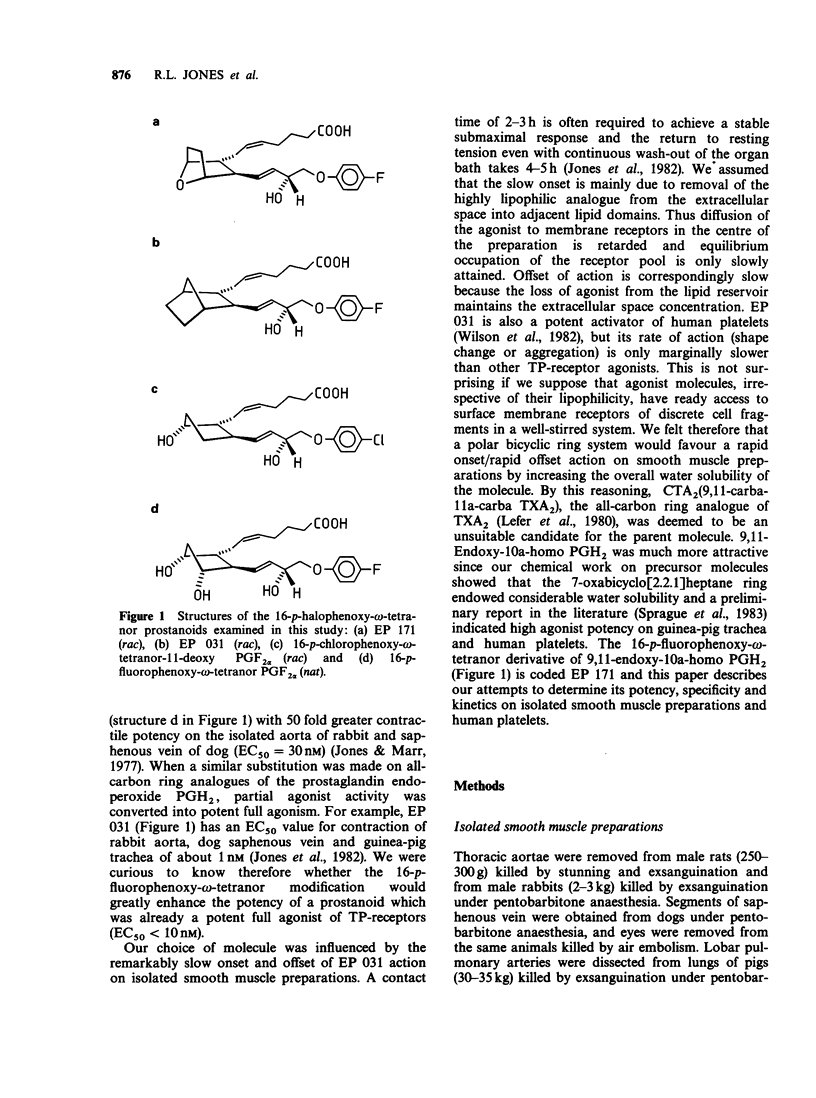
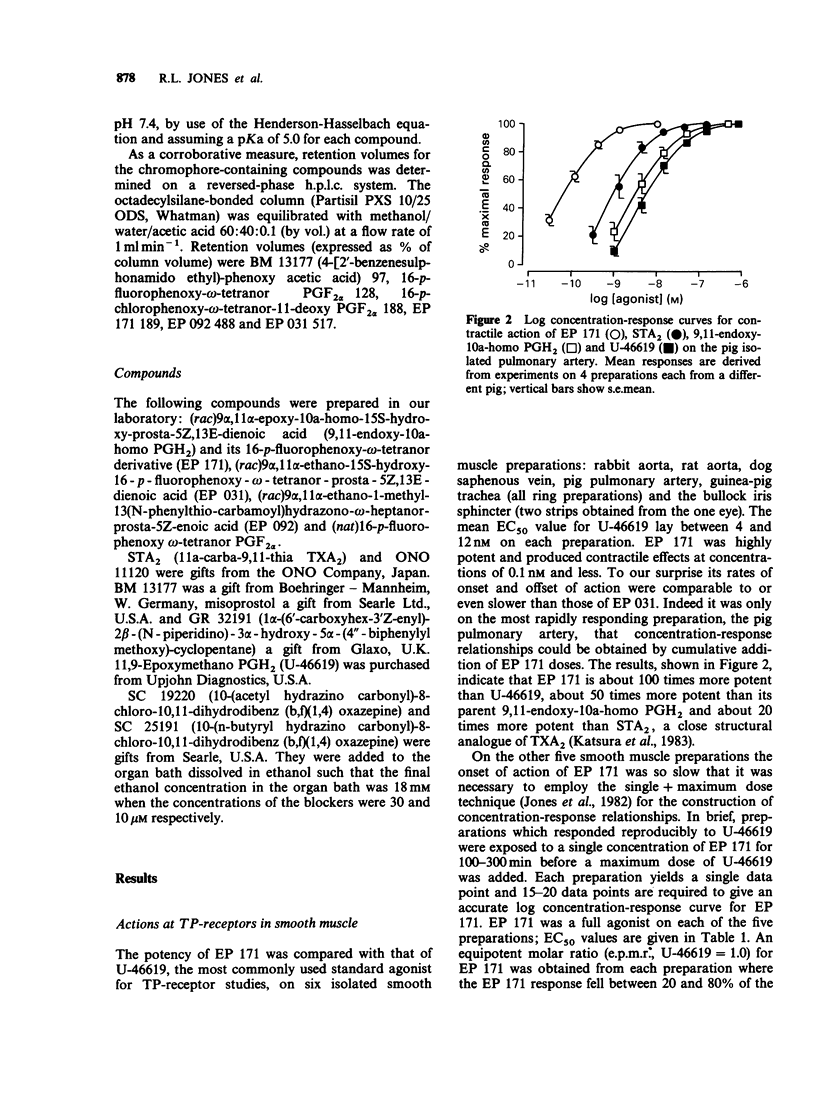
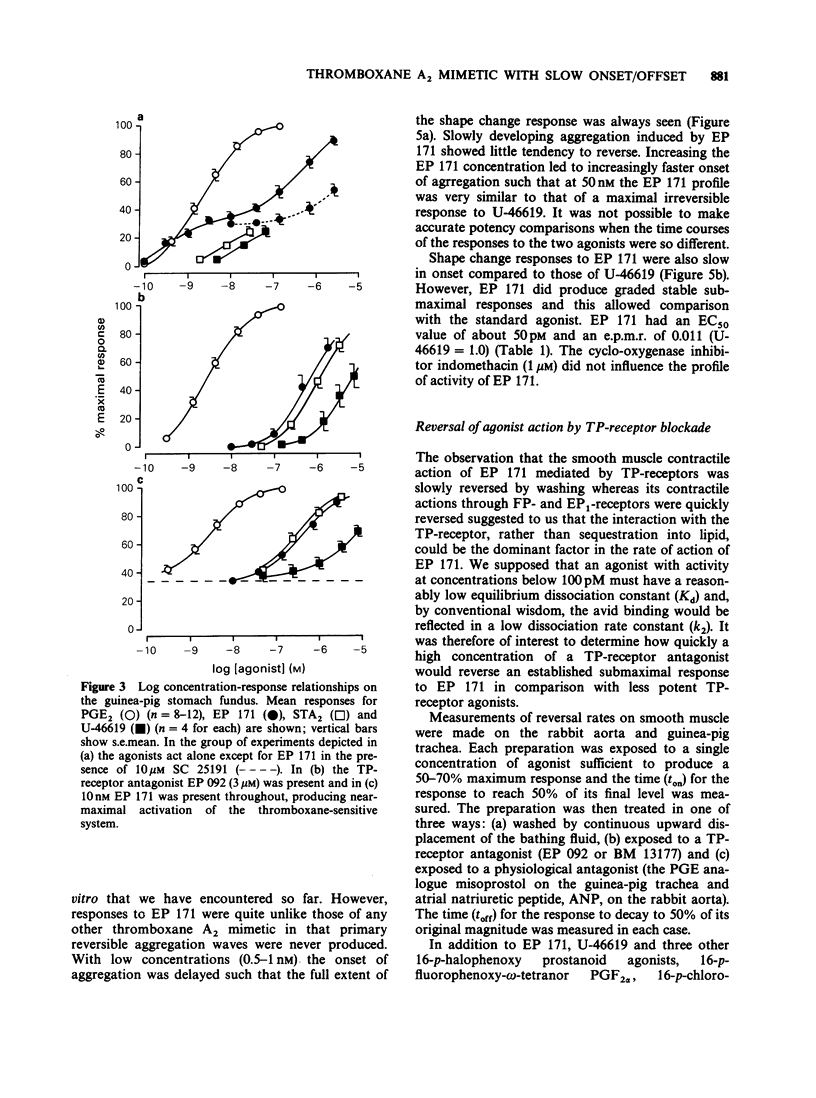
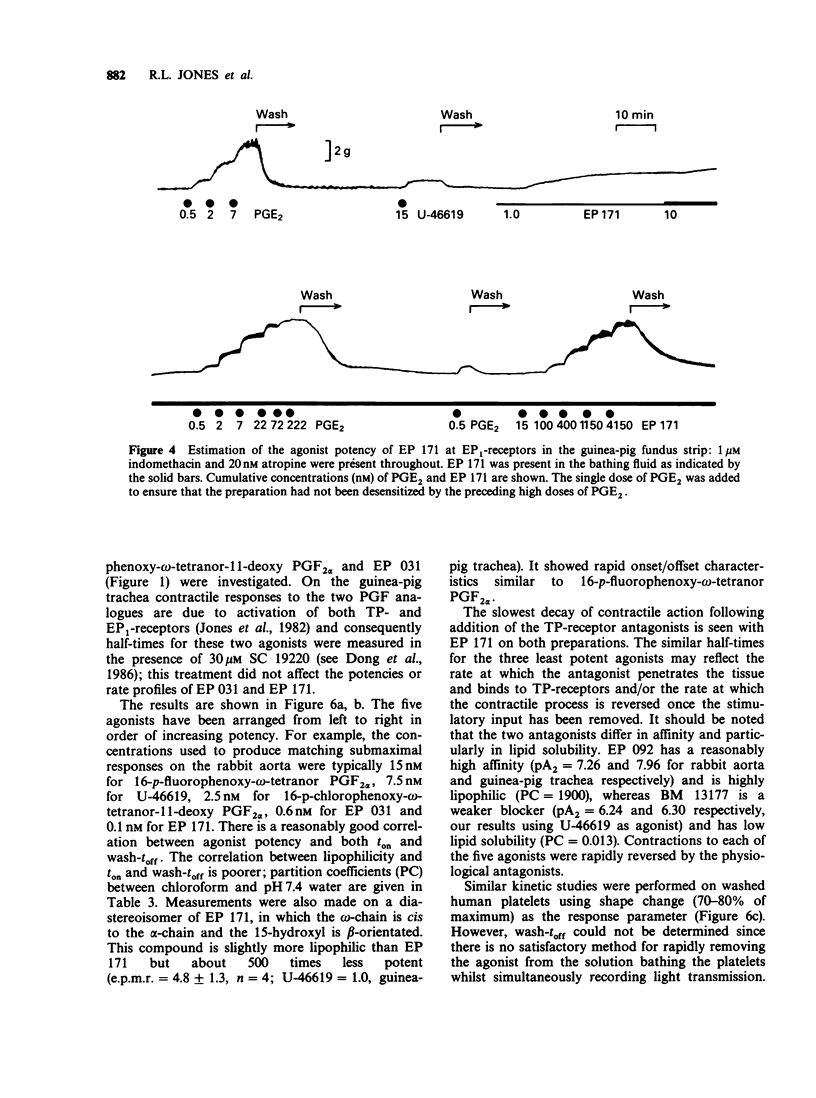
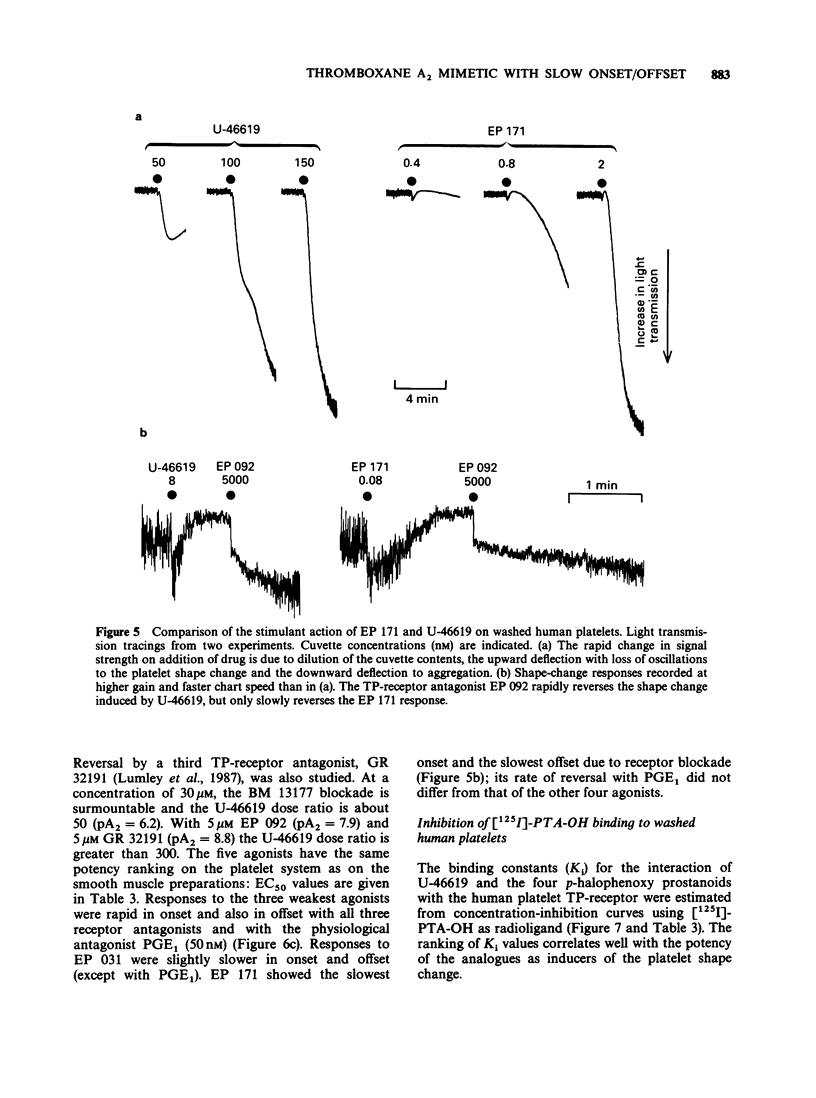
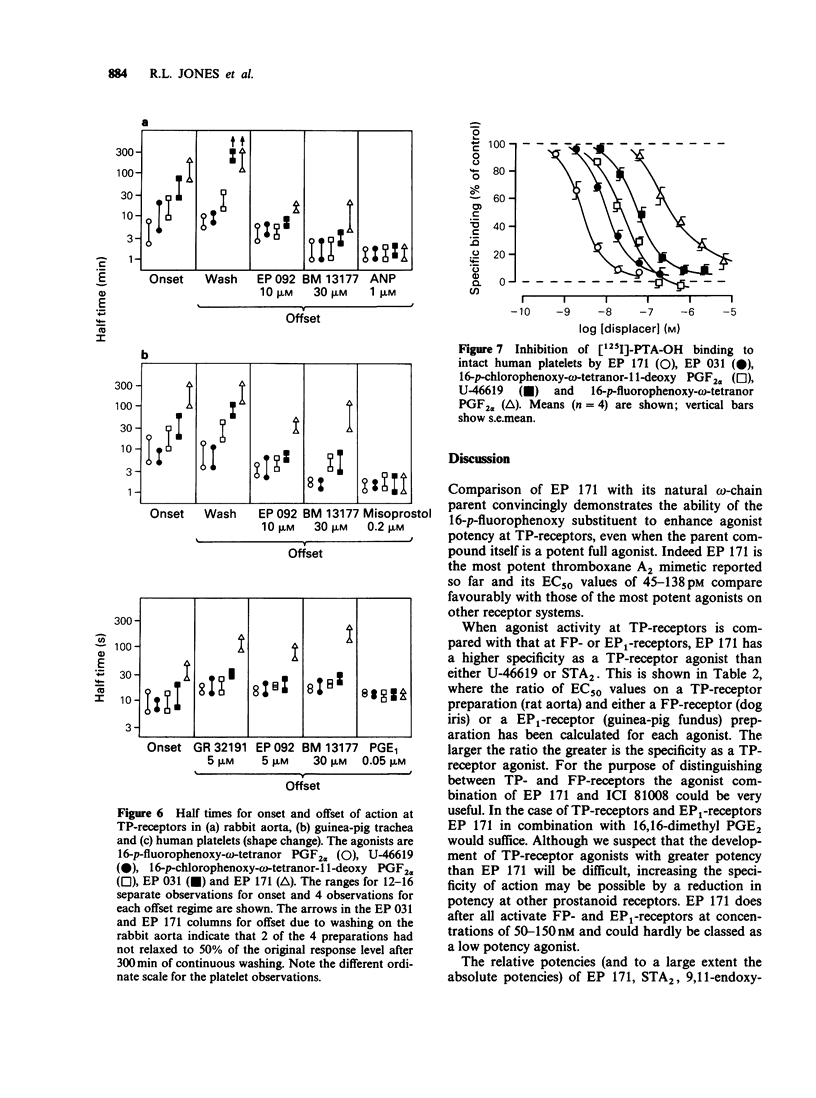
1. Replacement of the four-carbon omega-terminus in 9,11-endoxy-10a-homo prostaglandin H2 with a p-fluorophenoxy group produces a compound (EP 171) with very high agonist potency at TP-receptors. 2. On six isolated smooth muscle preparations EP 171 was 33-167 times more potent as a TP-receptor agonist than U-46619 (11,9-epoxymethano PGH2); EC50 values ranged from 45 to 138 pM. The actions of EP 171 were difficult to study because of their slow onset and offset. For example, on the guinea-pig trachea the time required for 50% reversal of EP 171-induced contractions during washout was about 3 h. 3. On the pig pulmonary artery, a more rapidly responding preparation, it was possible to show that the TP-receptor antagonist EP 092 blocked the contractile actions of EP 171 and U-46619 to similar extents: pA2 = 8.09 and 8.15 respectively. 4. EP 171 was also a very potent activator of human blood platelets, being about 90 times more potent than U-46619. Both shape change (0.1 nM) and aggregation (1 nM) were slow in onset, a profile not previously observed for a thromboxane A2-mimetic. 5. When potencies at TP-, EP1-(guinea-pig fundus) and FP-(dog iris sphincter) receptors were compared, EP 171 showed a higher specificity as a TP-receptor agonist than either STA2 or U-46619. These studies also showed that contrary to earlier reports, the guinea-pig fundus does contain TP-receptors mediating muscle contraction. However, the maximal response due to activation of TP-receptors was only about 35% of the PGE2 maximum. 6. Established responses to EP 171 were slowly reversed following addition of a high concentration of a TP-receptor antagonist (EP 092, GR 32191 or BM 13177). Faster reversals of three less potent 16-p-halophenoxy prostanoids and U-46619 were obtained. Half-times for offset (and onset) of agonist action appeared to correlate with potency rather than with lipophilicity. 7. Competition between the agonists and a radio iodinated PTA2 derivative ([125I]-PTA-OH) for binding to TP-receptors on intact human platelets was studied. IC50 values correlated well with aggregating potency, EP 171 having the lowest IC50 of 2.9 nM. The true Ki for EP 171 may be about 1 nM if both its racemic nature and reduction of initial free ligand concentration due to TP-receptor binding are taken into account.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R. A., Jones R. L., Peesapati V., Will S. G., Wilson N. H. Competitive antagonism at thromboxane receptors in human platelets. Br J Pharmacol. 1985 Mar;84(3):595–607. doi: 10.1111/j.1476-5381.1985.tb16139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R. A., Jones R. L., Wilson N. H. Ligand binding to thromboxane receptors on human platelets: correlation with biological activity. Br J Pharmacol. 1983 Aug;79(4):953–964. doi: 10.1111/j.1476-5381.1983.tb10541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Rate of offset of action of slow-acting muscarinic antagonists is fast. Nature. 1977 Nov 24;270(5635):354–356. doi: 10.1038/270354a0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Henderson R., Ritchie J. M. The binding of labelled tetrodotoxin to non-myelinated nerve fibres. J Physiol. 1972 Dec;227(1):95–126. doi: 10.1113/jphysiol.1972.sp010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Ritchie J. M. The kinetics of the interaction between tetrodotoxin and mammalian nonmyelinated nerve fibers. Mol Pharmacol. 1972 May;8(3):285–292. [PubMed] [Google Scholar]
- Dong Y. J., Jones R. L. Effects of prostaglandins and thromboxane analogues on bullock and dog iris sphincter preparations. Br J Pharmacol. 1982 May;76(1):149–155. doi: 10.1111/j.1476-5381.1982.tb09200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y. J., Jones R. L., Wilson N. H. Prostaglandin E receptor subtypes in smooth muscle: agonist activities of stable prostacyclin analogues. Br J Pharmacol. 1986 Jan;87(1):97–107. doi: 10.1111/j.1476-5381.1986.tb10161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner F. How fast do cardioactive steroids act independently of diffusion in guinea-pig myocardium? Br J Pharmacol. 1987 Aug;91(4):763–771. doi: 10.1111/j.1476-5381.1987.tb11274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Marr C. G. Actions of 16-aryloxy analogues of prostaglandin F2alpha on preparations responsive to prostaglandin endoperoxides. Br J Pharmacol. 1977 Dec;61(4):694–696. doi: 10.1111/j.1476-5381.1977.tb07563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Peesapati V., Wilson N. H. Antagonism of the thromboxane-sensitive contractile systems of the rabbit aorta, dog saphenous vein and guinea-pig trachea. Br J Pharmacol. 1982 Jul;76(3):423–438. doi: 10.1111/j.1476-5381.1982.tb09236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy I., Coleman R. A., Humphrey P. P., Levy G. P., Lumley P. Studies on the characterisation of prostanoid receptors: a proposed classification. Prostaglandins. 1982 Nov;24(5):667–689. doi: 10.1016/0090-6980(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Kennedy I., Coleman R. A., Humphrey P. P., Lumley P. Studies on the characterization of prostanoid receptors. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:327–332. [PubMed] [Google Scholar]
- Narumiya S., Okuma M., Ushikubi F. Binding of a radioiodinated 13-azapinane thromboxane antagonist to platelets: correlation with antiaggregatory activity in different species. Br J Pharmacol. 1986 Jun;88(2):323–331. doi: 10.1111/j.1476-5381.1986.tb10208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W. D., RANG H. P. THE UPTAKE OF ATROPINE AND RELATED DRUGS BY INTESTINAL SMOOTH MUSCLE OF THE GUINEA-PIG IN RELATION TO ACETYLCHOLINE RECEPTORS. Proc R Soc Lond B Biol Sci. 1965 Aug 24;163:1–44. doi: 10.1098/rspb.1965.0058. [DOI] [PubMed] [Google Scholar]
- Patscheke H., Stegmeier K. Investigation on a selective non-prostanoic thromboxane antagonist, BM 13.177, in human platelets. Thromb Res. 1984 Feb 1;33(3):277–288. doi: 10.1016/0049-3848(84)90163-4. [DOI] [PubMed] [Google Scholar]
- Rang H. P. The kinetics of action of acetylcholine antagonists in smooth muscle. Proc R Soc Lond B Biol Sci. 1966 Apr 19;164(996):488–510. doi: 10.1098/rspb.1966.0045. [DOI] [PubMed] [Google Scholar]
- Sprague P. W., Heikes J. E., Gougoutas J. Z., Malley M. F., Harris D. N., Greenberg R. Synthesis and in vitro pharmacology of 7-oxabicyclo[2.2.1]heptane analogues of thromboxane A2/PGH2. J Med Chem. 1985 Nov;28(11):1580–1590. doi: 10.1021/jm00149a007. [DOI] [PubMed] [Google Scholar]
- Sprague P. W., Heikes J. E., Harris D. N., Greenberg R. 7-Oxabicyclo[2.2.1]heptane analogs as modulators of the thromboxane A2 and prostacyclin receptors. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:337–343. [PubMed] [Google Scholar]
- Stürzebecher S., Haberey M., Müller B., Schillinger E., Schröder G., Skuballa W., Stock G., Vorbrüggen H., Witt W. Pharmacological profile of a novel carbacyclin derivative with high metabolic stability and oral activity in the rat. Prostaglandins. 1986 Jan;31(1):95–109. doi: 10.1016/0090-6980(86)90228-5. [DOI] [PubMed] [Google Scholar]
- Wilson N. H., Peesapati V., Jones R. L., Hamilton K. Synthesis of prostanoids with bicyclo[2.2.1]heptane, bicyclo[3.1.1]heptane, and bicyclo[2.2.2]octane ring systems. Activities of 15-hydroxy epimers on human platelets. J Med Chem. 1982 May;25(5):495–500. doi: 10.1021/jm00347a004. [DOI] [PubMed] [Google Scholar]


