Abstract
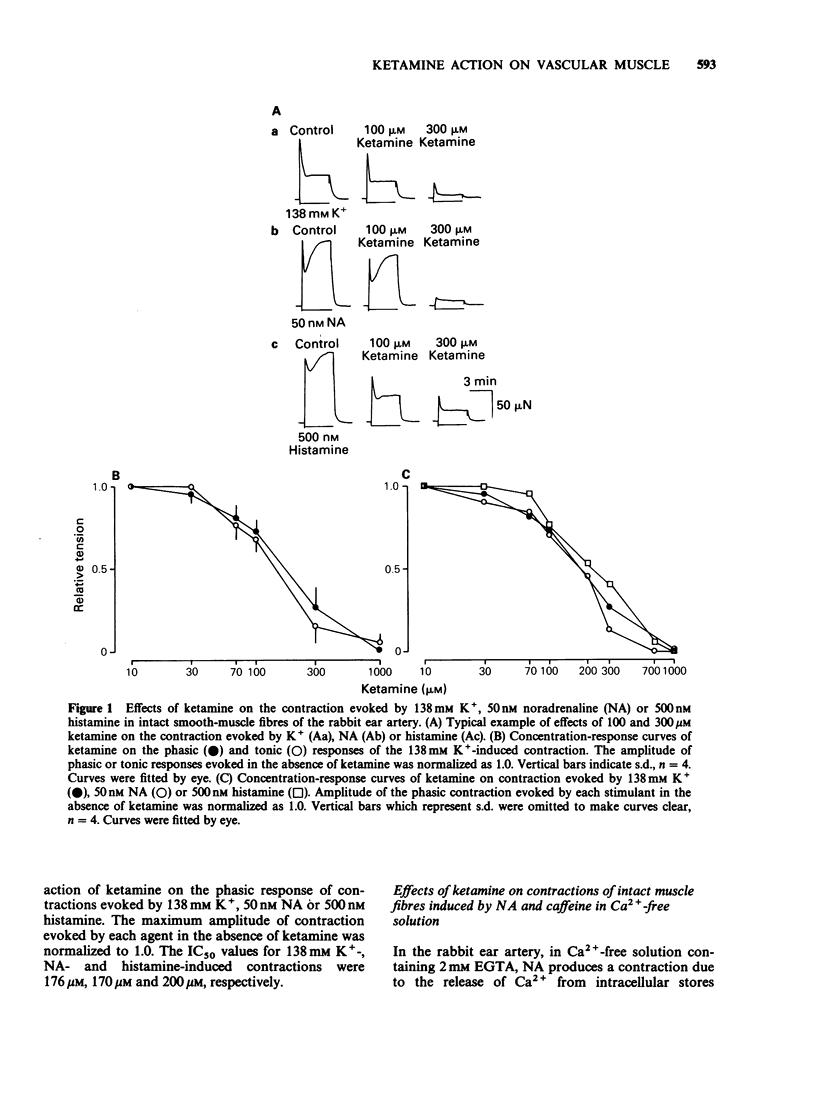
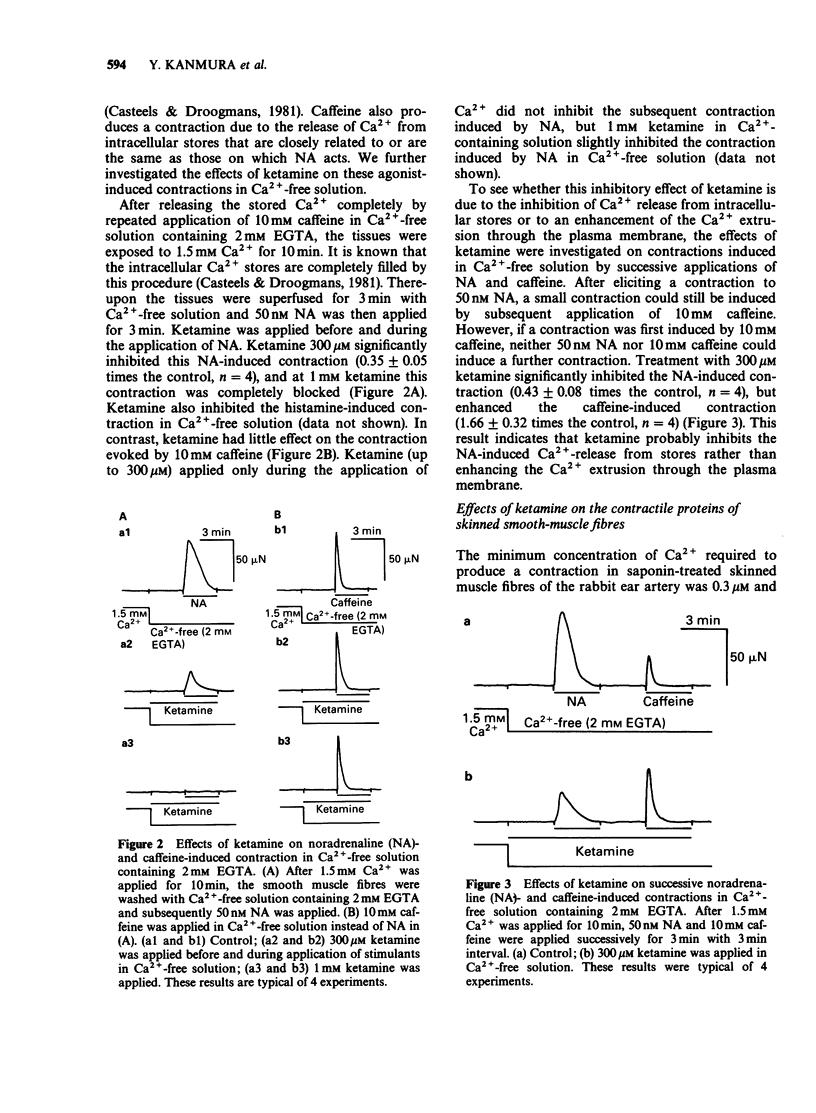
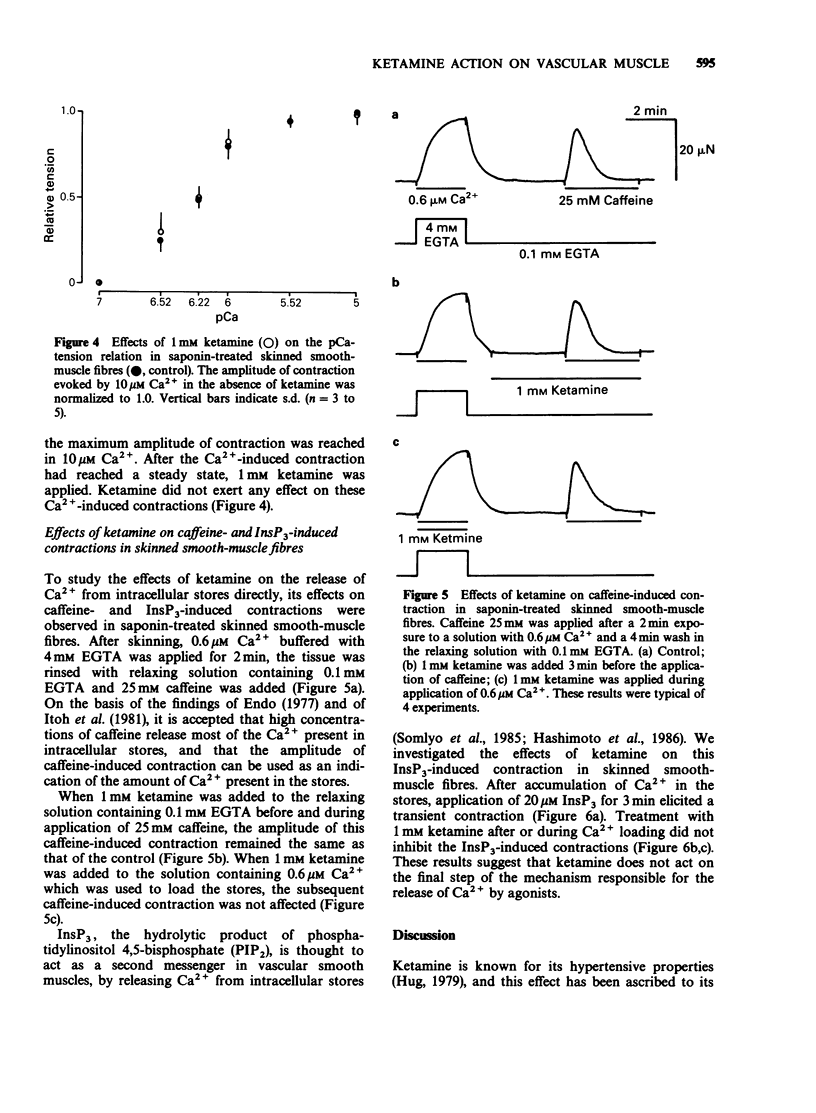
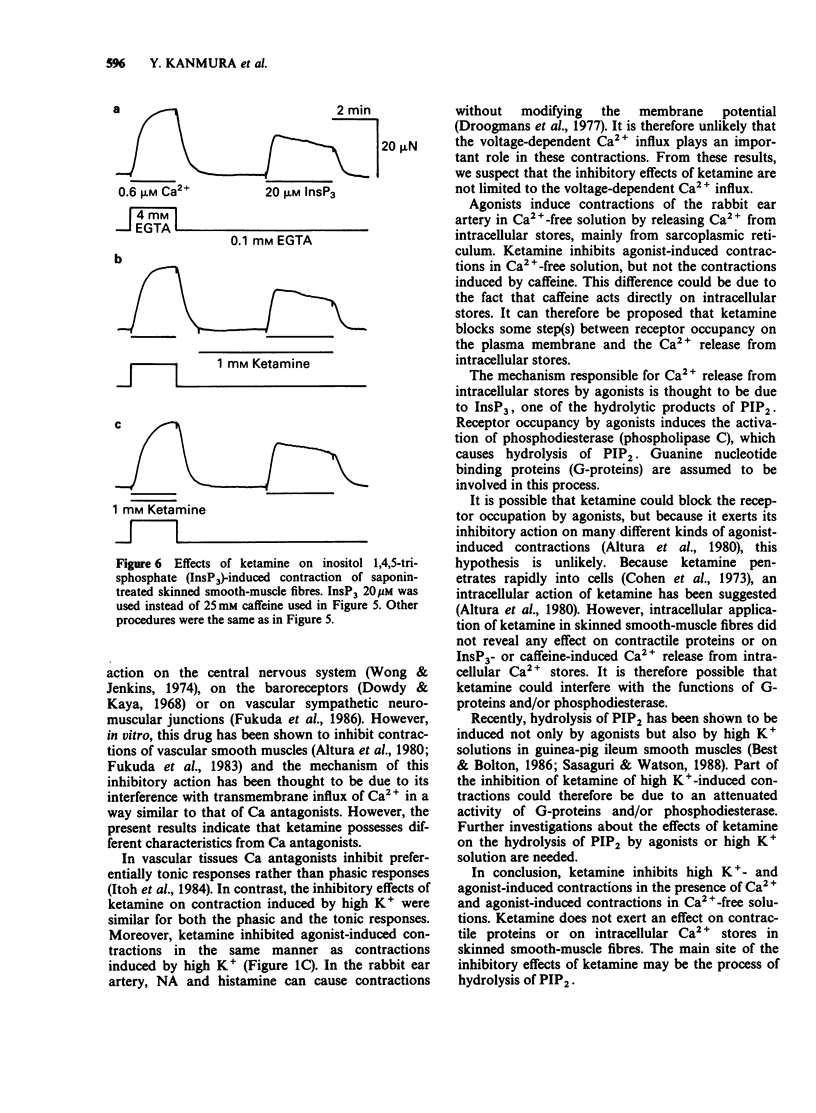
1. The effects of ketamine, an intravenous anaesthetic, on the rabbit ear artery were investigated by measuring the tension in intact and saponin-treated skinned smooth-muscle fibres. 2. Ketamine dose-dependently inhibited contractions of intact smooth-muscle fibres induced by high K+ solution and by noradrenaline (NA) or histamine in Krebs solution. This drug similarly attenuated both phasic and tonic contractions induced by high K+ solution. 3. Ketamine also inhibited NA- or histamine-induced contractions in Ca2+-free solution containing 2mM EGTA, but it did not affect the caffeine-induced contraction in this solution. 4. Because the pCa-tension relationship of saponin-treated skinned smooth-muscle fibres was not affected, it can be proposed that ketamine does not have an effect on the contractile proteins. 5. In the presence of 5mM NaN3, 20 microM inositol 1,4,5-trisphosphate (InsP3) or 25mM caffeine produced a contraction in skinned smooth-muscle fibres after accumulation of Ca2+ by intracellular stores. Analysis of the InsP3- or caffeine-induced contractions indicates that ketamine does not have an effect on the Ca2+ accumulation into and Ca2+ release from the intracellular stores. 6. These results indicate that the relaxant effects produced by ketamine in the rabbit ear artery are not likely to be due to an intracellular action. The inhibitory effects of ketamine could be caused by a decrease of the Ca2+ influx through the plasma membrane or interference with the process of signal transduction between receptors on the plasma membrane and intracellular stores.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altura B. M., Altura B. T., Carella A. Effects of ketamine on vascular smooth muscle function. Br J Pharmacol. 1980 Oct;70(2):257–267. doi: 10.1111/j.1476-5381.1980.tb07931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best L., Bolton T. B. Depolarisation of guinea-pig visceral smooth muscle causes hydrolysis of inositol phospholipids. Naunyn Schmiedebergs Arch Pharmacol. 1986 May;333(1):78–82. doi: 10.1007/BF00569664. [DOI] [PubMed] [Google Scholar]
- Casteels R., Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981 Aug;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clanachan A. S., McGrath J. C., MacKenzie J. E. Cardiovascular effects of ketamine in the pithed rat, rabbit and cat. Br J Anaesth. 1976 Oct;48(10):935–939. doi: 10.1093/bja/48.10.935. [DOI] [PubMed] [Google Scholar]
- Cohen M. L., Chan S. L., Way W. L., Trevor A. J. Distribution in the brain and metabolism of ketamine in the rat after intravenous administration. Anesthesiology. 1973 Oct;39(4):370–376. doi: 10.1097/00000542-197310000-00003. [DOI] [PubMed] [Google Scholar]
- DOMINO E. F., CHODOFF P., CORSSEN G. PHARMACOLOGIC EFFECTS OF CI-581, A NEW DISSOCIATIVE ANESTHETIC, IN MAN. Clin Pharmacol Ther. 1965 May-Jun;6:279–291. doi: 10.1002/cpt196563279. [DOI] [PubMed] [Google Scholar]
- Dowdy E. G., Kaya K. Studies of the mechanism of cardiovascular responses to CI-581. Anesthesiology. 1968 Sep-Oct;29(5):931–943. doi: 10.1097/00000542-196809000-00014. [DOI] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fukuda S., Murakawa T., Takeshita H., Toda N. Direct effects of ketamine on isolated canine cerebral and mesenteric arteries. Anesth Analg. 1983 Jun;62(6):553–558. [PubMed] [Google Scholar]
- Fukuda S., Su C., Lee T. J. Potentiation of pressor responses to serotonin by ketamine in isolated perfused rat mesentery. J Cardiovasc Pharmacol. 1986 Jul-Aug;8(4):765–770. [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1982 Jan 29;104(2):830–836. doi: 10.1016/0006-291x(82)90712-4. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Itoh T., Kanmura Y., Kuriyama H. Inositol 1,4,5-trisphosphate activates pharmacomechanical coupling in smooth muscle of the rabbit mesenteric artery. J Physiol. 1986 Jan;370:605–618. doi: 10.1113/jphysiol.1986.sp015953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Tension responses of chemically skinned fibre bundles of the guinea-pig taenia caeci under varied ionic environments. J Physiol. 1981 Nov;320:449–467. doi: 10.1113/jphysiol.1981.sp013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H. A23187 increases calcium permeability of store sites more than of surface membranes in the rabbit mesenteric artery. J Physiol. 1985 Feb;359:467–484. doi: 10.1113/jphysiol.1985.sp015597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H. Inorganic phosphate regulates the contraction-relaxation cycle in skinned muscles of the rabbit mesenteric artery. J Physiol. 1986 Jul;376:231–252. doi: 10.1113/jphysiol.1986.sp016151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H., Suzuki H. Nisoldipine-induced relaxation in intact and skinned smooth muscles of rabbit coronary arteries. Br J Pharmacol. 1984 Sep;83(1):243–258. doi: 10.1111/j.1476-5381.1984.tb10141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Excitation--contraction coupling in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1981 Dec;321:513–535. doi: 10.1113/jphysiol.1981.sp014000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmura Y., Missiaen L., Casteels R. Properties of intracellular calcium stores in pregnant rat myometrium. Br J Pharmacol. 1988 Sep;95(1):284–290. doi: 10.1111/j.1476-5381.1988.tb16575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Ito Y., Suzuki H., Kitamura K., Itoh T. Factors modifying contraction-relaxation cycle in vascular smooth muscles. Am J Physiol. 1982 Nov;243(5):H641–H662. doi: 10.1152/ajpheart.1982.243.5.H641. [DOI] [PubMed] [Google Scholar]
- Sasaguri T., Watson S. P. Lowering of the extracellular Na+ concentration enhances high-K+-induced formation of inositol phosphates in the guinea-pig ileum. Biochem J. 1988 Jun 15;252(3):883–888. doi: 10.1042/bj2520883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Bond M., Somlyo A. P., Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5231–5235. doi: 10.1073/pnas.82.15.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtue R. W., Alanis J. M., Mori M., Lafargue R. T., Vogel J. H., Metcalf D. R. An anesthetic agent: 2-orthochlorophenyl, 2-methylamino cyclohexanone HCl (CI-581). Anesthesiology. 1967 Sep-Oct;28(5):823–833. doi: 10.1097/00000542-196709000-00008. [DOI] [PubMed] [Google Scholar]
- Wong D. H., Jenkins L. C. An experimental study of the mechanism of action of ketamine on the central nervous system. Can Anaesth Soc J. 1974 Jan;21(1):57–67. doi: 10.1007/BF03004579. [DOI] [PubMed] [Google Scholar]


