Abstract
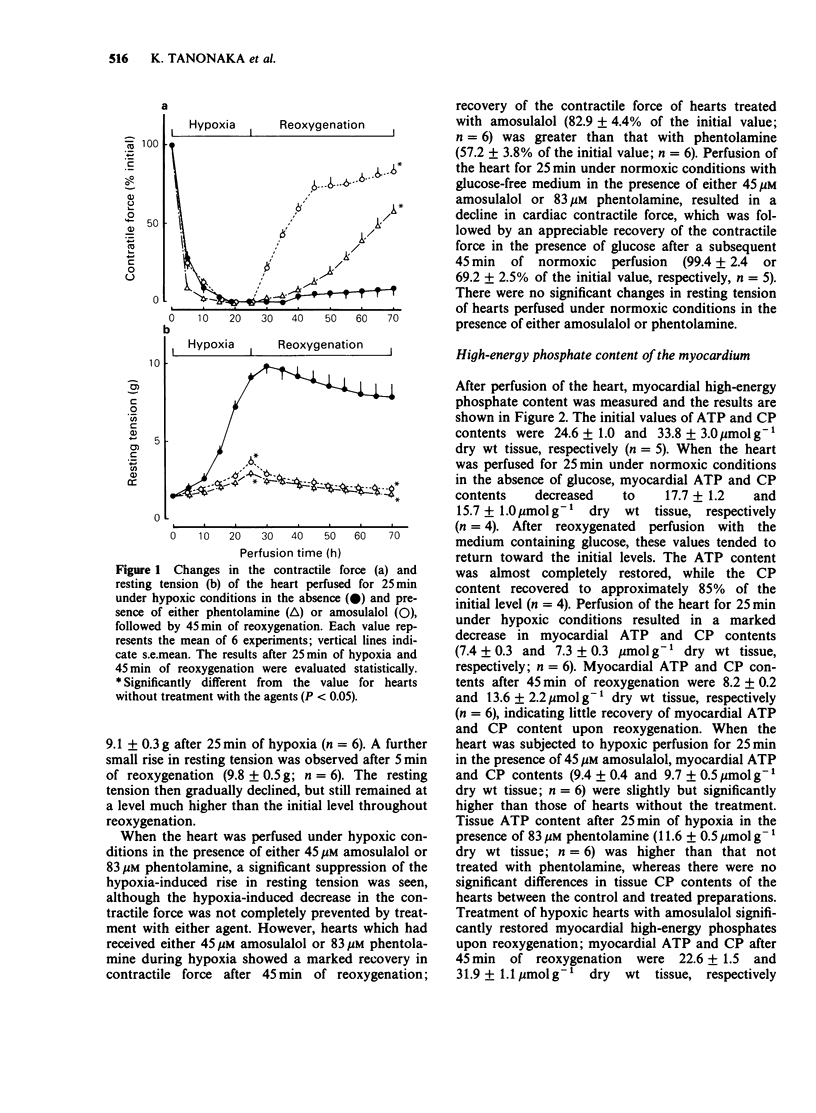
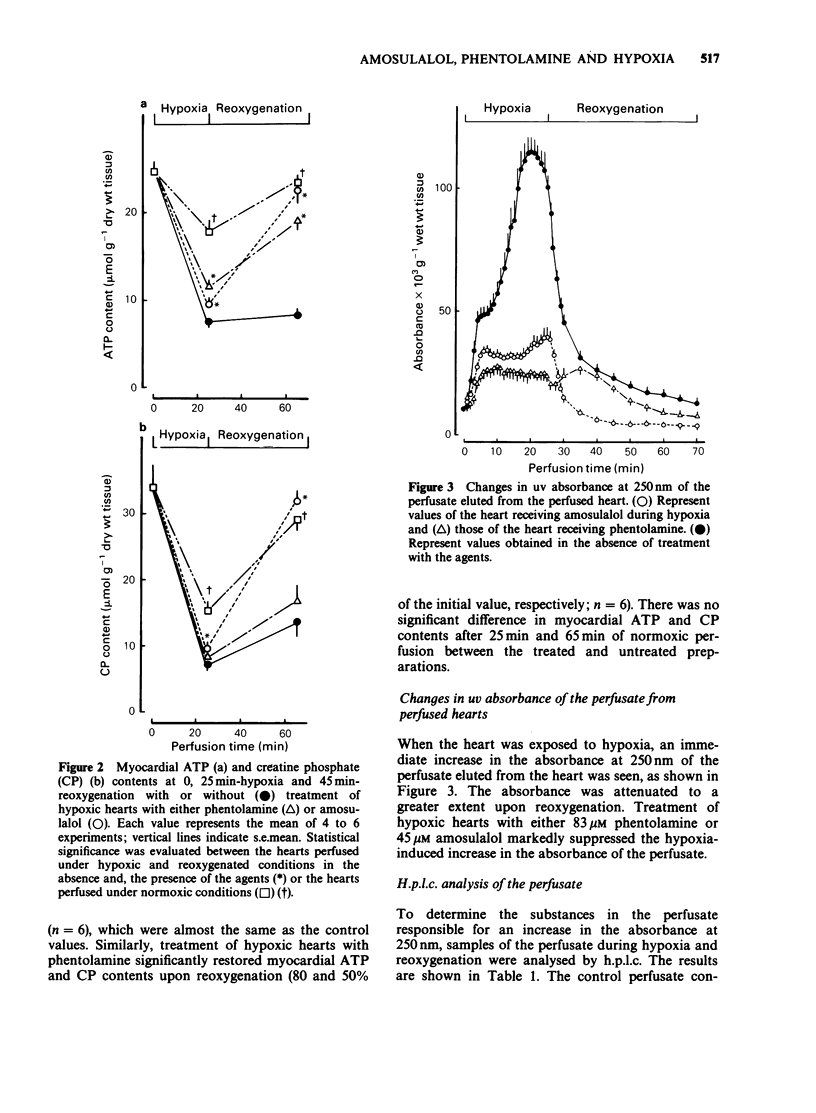
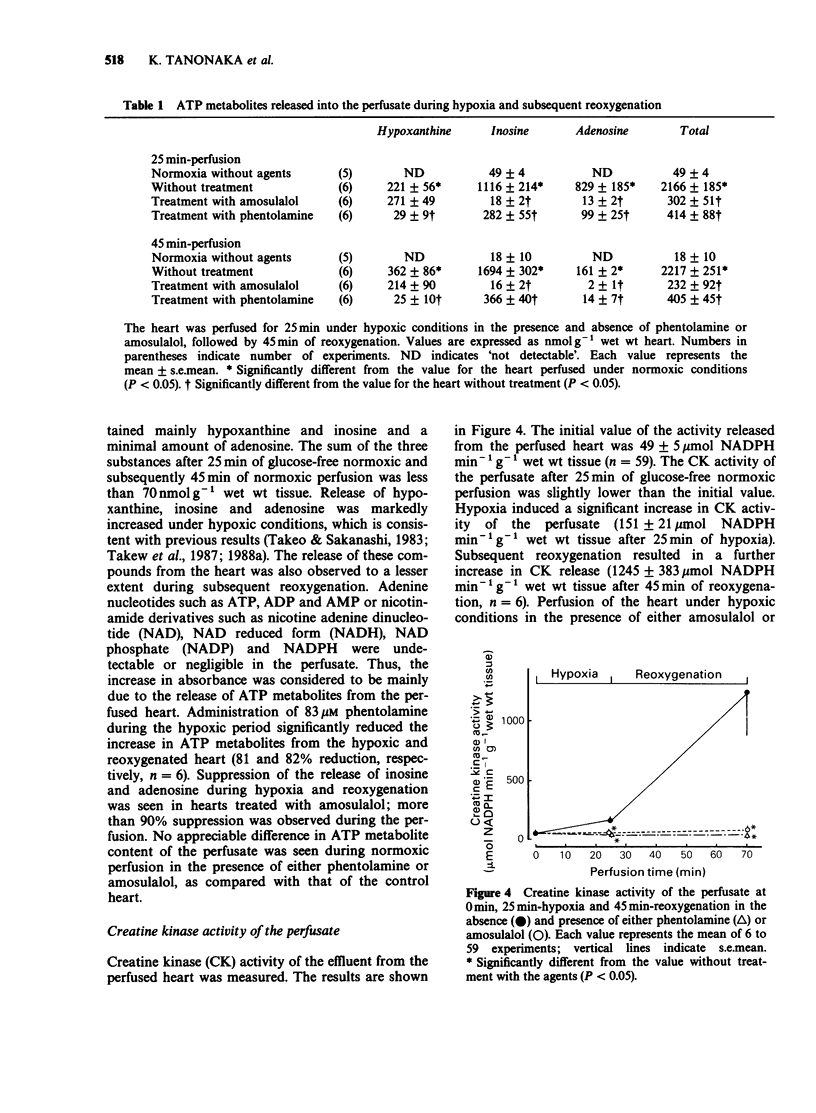
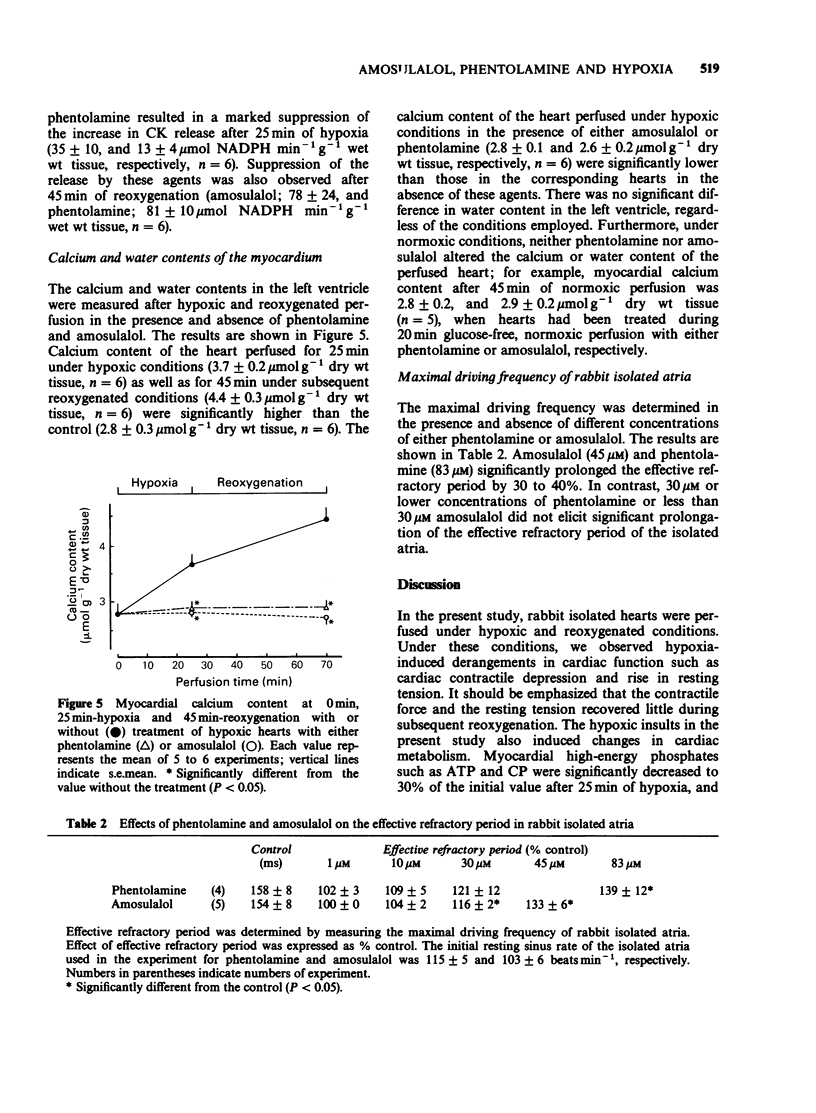
1. The effects of phentolamine, an alpha-adrenoceptor blocking agent and amousulalol, an alpha 1 and beta-adrenoceptor antagonist on hypoxia-induced impairment in cardiac function and metabolism were examined using the isolated heart Langendorff preparation of the rabbit. 2. Hypoxia induced cessation of cardiac contractile force, a rise in resting tension, a decrease in myocardial high-energy phosphates, an increase in tissue calcium content and the release of ATP metabolites from the heart. Subsequent reoxygenation resulted in little recovery of cardiac contractile force, and there were further increases in tissue calcium content and in the release of creatine kinase from the heart. 3. Treatment of hypoxic hearts with either 83 microM phentolamine or 45 microM amosulalol resulted in a suppression of the rise in resting tension, the tissue calcium accumulation and the release of creatine kinase and ATP metabolites during hypoxia. This treatment also elicited significant recovery of cardiac contractile force, restoration of myocardial high-energy phosphates, suppression of the release of creatine kinase and the accumulation of tissue calcium during reoxygenation. Both 83 microM phentolamine and 45 microM amosulalol a significant prolongation of the effective refractory period of rabbit isolated atria. 4. Lower concentrations of phentolamine (16 microM) and amosulalol) (9 microM), which are sufficient to exert an alpha-adrenoceptor blocking action, did not elicit an appreciable effect on the post-hypoxic recovery of cardiac contractile force. 5. These results suggest that phentolamine and amosulalol are capable of protecting the myocardium from hypoxia-induced derangements in cardiac function and metabolism. This effect is probably attributable to their membrane stabilizing effect, rather than to their alpha-adrenoceptor blocking action.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Orchard C. H. Myocardial contractile function during ischemia and hypoxia. Circ Res. 1987 Feb;60(2):153–168. doi: 10.1161/01.res.60.2.153. [DOI] [PubMed] [Google Scholar]
- Ashraf M., Rahamathulla P. M. Cardiac injury in short duration anoxia and modification by diltiazem, a calcium channel blocking agent. J Am Coll Cardiol. 1984 May;3(5):1237–1244. doi: 10.1016/s0735-1097(84)80182-5. [DOI] [PubMed] [Google Scholar]
- Benfey B. G., Varma D. R. Antisympathomimetic and antifibrillatory effects of pronethalol and propranolol. Br J Pharmacol Chemother. 1966 Jan;26(1):3–8. doi: 10.1111/j.1476-5381.1966.tb01804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunwald E., Kloner R. A. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation. 1982 Dec;66(6):1146–1149. doi: 10.1161/01.cir.66.6.1146. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Kaltenbach J. P. Oxygen-induced enzyme release: early events and a proposed mechanism. J Mol Cell Cardiol. 1979 Apr;11(4):389–406. doi: 10.1016/0022-2828(79)90425-5. [DOI] [PubMed] [Google Scholar]
- Goldhaber S. Z., Pohost G. M., Kloner R. A., Andrews E., Newell J. B., Ingwall J. S. Inosine: a protective agent in an organ culture model of myocardial ischemia. Circ Res. 1982 Aug;51(2):181–188. doi: 10.1161/01.res.51.2.181. [DOI] [PubMed] [Google Scholar]
- Grochowski E. C., Ganote C. E., Hill M. L., Jennings R. B. Experimental myocardial ischemic injury. I. A comparison of Stadie-Riggs and free-hand slicing techniques on tissue ultrastructure, water and electrolytes during in vitro incubation. J Mol Cell Cardiol. 1976 Mar;8(3):173–187. doi: 10.1016/0022-2828(76)90035-3. [DOI] [PubMed] [Google Scholar]
- Harmsen E., de Tombe P. P., de Jong J. W., Achterberg P. W. Enhanced ATP and GTP synthesis from hypoxanthine or inosine after myocardial ischemia. Am J Physiol. 1984 Jan;246(1 Pt 2):H37–H43. doi: 10.1152/ajpheart.1984.246.1.H37. [DOI] [PubMed] [Google Scholar]
- Harmsen E., de Tombe P. P., de Jong J. W., Achterberg P. W. Enhanced ATP and GTP synthesis from hypoxanthine or inosine after myocardial ischemia. Am J Physiol. 1984 Jan;246(1 Pt 2):H37–H43. doi: 10.1152/ajpheart.1984.246.1.H37. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Garlick P. B., Humphrey S. M. Ischemic contracture of the myocardium: mechanisms and prevention. Am J Cardiol. 1977 Jun;39(7):986–993. doi: 10.1016/s0002-9149(77)80212-9. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Chain E. B. Abrupt reoxygenation of the anoxic potassium-arrested perfused rat heart: a study of myocardial enzyme release. J Mol Cell Cardiol. 1973 Aug;5(4):395–407. doi: 10.1016/0022-2828(73)90030-8. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M. Enzyme release during myocardial anoxia: a study of metabolic protection. J Mol Cell Cardiol. 1975 Jul;7(7):463–482. doi: 10.1016/0022-2828(75)90164-9. [DOI] [PubMed] [Google Scholar]
- Hearse D. J. Reperfusion of the ischemic myocardium. J Mol Cell Cardiol. 1977 Aug;9(8):605–616. doi: 10.1016/s0022-2828(77)80357-x. [DOI] [PubMed] [Google Scholar]
- Honda K., Takenaka T., Miyata-Osawa A., Terai M. Adrenoceptor blocking properties of the stereoisomers of amosulalol (YM-09538) and the corresponding desoxy derivative (YM-11133). J Pharmacol Exp Ther. 1986 Mar;236(3):776–783. [PubMed] [Google Scholar]
- Jarmakani J. M., Nakanishi T., Jarmakani R. N. Effect of hypoxia on calcium exchange in neonatal mammalian myocardium. Am J Physiol. 1979 Nov;237(5):H612–H619. doi: 10.1152/ajpheart.1979.237.5.H612. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Hawkins H. K., Lowe J. E., Hill M. L., Klotman S., Reimer K. A. Relation between high energy phosphate and lethal injury in myocardial ischemia in the dog. Am J Pathol. 1978 Jul;92(1):187–214. [PMC free article] [PubMed] [Google Scholar]
- Jennings R. B., Reimer K. A., Steenbergen C. Myocardial ischemia revisited. The osmolar load, membrane damage, and reperfusion. J Mol Cell Cardiol. 1986 Aug;18(8):769–780. doi: 10.1016/s0022-2828(86)80952-x. [DOI] [PubMed] [Google Scholar]
- Murry C. E., Jennings R. B., Reimer K. A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986 Nov;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Nakahara T., Takeo S. Irreversible changes in oxidative phosphorylation activity of the mitochondrial membrane from hearts subjected to hypoxia and reoxygenation. Can J Cardiol. 1986 Jan-Feb;2(1):24–33. [PubMed] [Google Scholar]
- Nayler W. G. Calcium and cell death. Eur Heart J. 1983 May;4 (Suppl 100):33–41. doi: 10.1093/eurheartj/4.suppl_c.33. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Ferrari R., Poole-Wilson P. A., Yepez C. E. A protective effect of a mild acidosis on hypoxic heart muscle. J Mol Cell Cardiol. 1979 Oct;11(10):1053–1071. doi: 10.1016/0022-2828(79)90394-8. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Gordon M., Stephens D. J., Sturrock W. J. The protective effect of prazosin on the ischaemic and reperfused myocardium. J Mol Cell Cardiol. 1985 Jul;17(7):685–699. doi: 10.1016/s0022-2828(85)80068-7. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Perry S. E., Elz J. S., Daly M. J. Calcium, sodium, and the calcium paradox. Circ Res. 1984 Aug;55(2):227–237. doi: 10.1161/01.res.55.2.227. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Poole-Wilson P. A., Williams A. Hypoxia and calcium. J Mol Cell Cardiol. 1979 Jul;11(7):683–706. doi: 10.1016/0022-2828(79)90381-x. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Yepez C. E., Poole-Wilson P. A. The effect of beta-adrenoceptor and Ca2+ antagonist drugs on the hypoxia-induced increased in resting tension. Cardiovasc Res. 1978 Nov;12(11):666–674. doi: 10.1093/cvr/12.11.666. [DOI] [PubMed] [Google Scholar]
- Northover B. J. A comparison of the electrophysiological actions of phentolamine with those of some other antiarrhythmic drugs on tissues isolated from the rat heart. Br J Pharmacol. 1983 Sep;80(1):85–93. doi: 10.1111/j.1476-5381.1983.tb11053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reibel D. K., Rovetto M. J. Myocardial ATP synthesis and mechanical function following oxygen deficiency. Am J Physiol. 1978 May;234(5):H620–H624. doi: 10.1152/ajpheart.1978.234.5.H620. [DOI] [PubMed] [Google Scholar]
- Schrader J., Haddy F. J., Gerlach E. Release of adenosine, inosine and hypoxanthine from the isolated guinea pig heart during hypoxia, flow-autoregulation and reactive hyperemia. Pflugers Arch. 1977 May 6;369(1):1–6. doi: 10.1007/BF00580802. [DOI] [PubMed] [Google Scholar]
- Sharma A. D., Saffitz J. E., Lee B. I., Sobel B. E., Corr P. B. Alpha adrenergic-mediated accumulation of calcium in reperfused myocardium. J Clin Invest. 1983 Sep;72(3):802–818. doi: 10.1172/JCI111051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T. Comparison of pre- and postsynaptic alpha-adrenoceptor blocking effects of E-643 in the isolated vas deferens of the rat. Jpn J Pharmacol. 1981 Jun;31(3):361–368. doi: 10.1254/jjp.31.361. [DOI] [PubMed] [Google Scholar]
- Takenaka T., Asano M., Berdeaux A., Giudicelli J. F. Adrenoceptor blocking hemodynamic and coronary effects of YM-09538, a new combined alpha- and beta-adrenoceptor blocking drug, in anesthetized dogs. Eur J Pharmacol. 1982 Nov 5;85(1):35–50. doi: 10.1016/0014-2999(82)90422-8. [DOI] [PubMed] [Google Scholar]
- Takenaka T., Honda K., Fujikura T., Niigata K., Tachikawa S., Inukai N. New sulfamoylphenethylamines, potent alpha 1-adrenoceptor antagonists. J Pharm Pharmacol. 1984 Aug;36(8):539–542. doi: 10.1111/j.2042-7158.1984.tb04447.x. [DOI] [PubMed] [Google Scholar]
- Takeo S., Sakanashi M. Possible mechanisms for reoxygenation-induced recovery of myocardial high-energy phosphates after hypoxia. J Mol Cell Cardiol. 1983 Sep;15(9):577–594. doi: 10.1016/0022-2828(83)90268-7. [DOI] [PubMed] [Google Scholar]
- Takeo S., Tanonaka K., Miyake K., Fukumoto T. Role of ATP metabolites in induction of incomplete recovery of cardiac contractile force after hypoxia. Can J Cardiol. 1988 May;4(4):193–200. [PubMed] [Google Scholar]
- Takeo S., Tanonaka K., Miyake K., Imago M. Adenine nucleotide metabolites are beneficial for recovery of cardiac contractile force after hypoxia. J Mol Cell Cardiol. 1988 Mar;20(3):187–199. doi: 10.1016/s0022-2828(88)80052-x. [DOI] [PubMed] [Google Scholar]
- Takeo S., Tanonaka K., Tazuma Y., Fukao N., Yoshikawa C., Fukumoto T., Tanaka T. Diltiazem and verapamil reduce the loss of adenine nucleotide metabolites from hypoxic hearts. J Mol Cell Cardiol. 1988 May;20(5):443–456. doi: 10.1016/s0022-2828(88)80136-6. [DOI] [PubMed] [Google Scholar]
- Takeo S., Tanonaka K., Tazuma Y., Miyake K., Murai R. Possible mechanism by which coenzyme Q10 improves reoxygenation-induced recovery of cardiac contractile force after hypoxia. J Pharmacol Exp Ther. 1987 Dec;243(3):1131–1138. [PubMed] [Google Scholar]
- Vary T. C., Angelakos E. T., Schaffer S. W. Relationship between adenine nucleotide metabolism and irreversible ischemic tissue damage in isolated perfused rat heart. Circ Res. 1979 Aug;45(2):218–225. doi: 10.1161/01.res.45.2.218. [DOI] [PubMed] [Google Scholar]


