Abstract
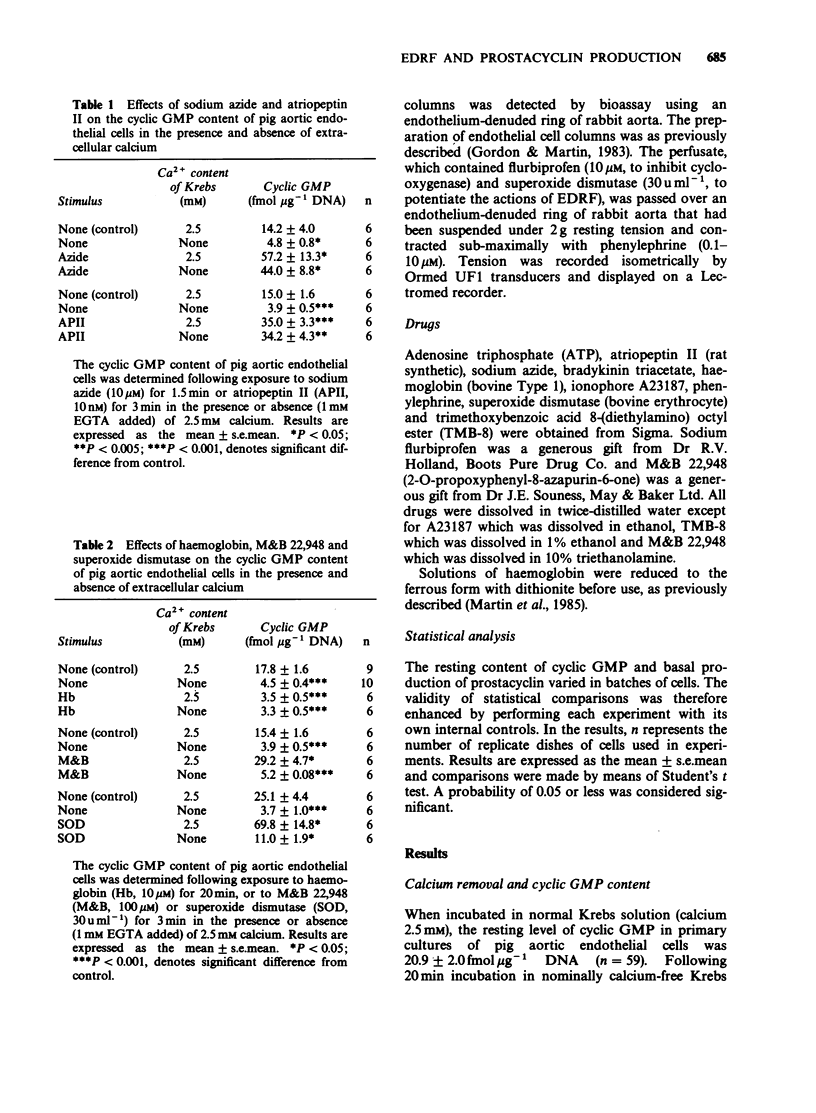
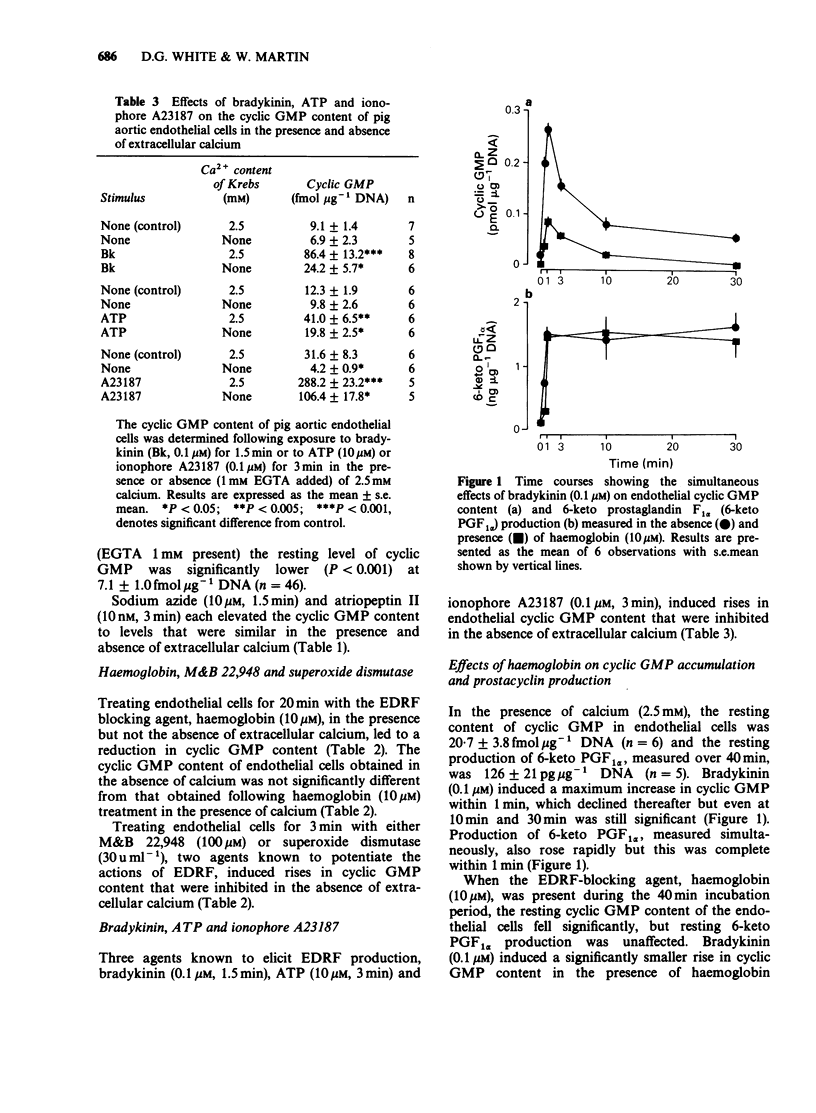
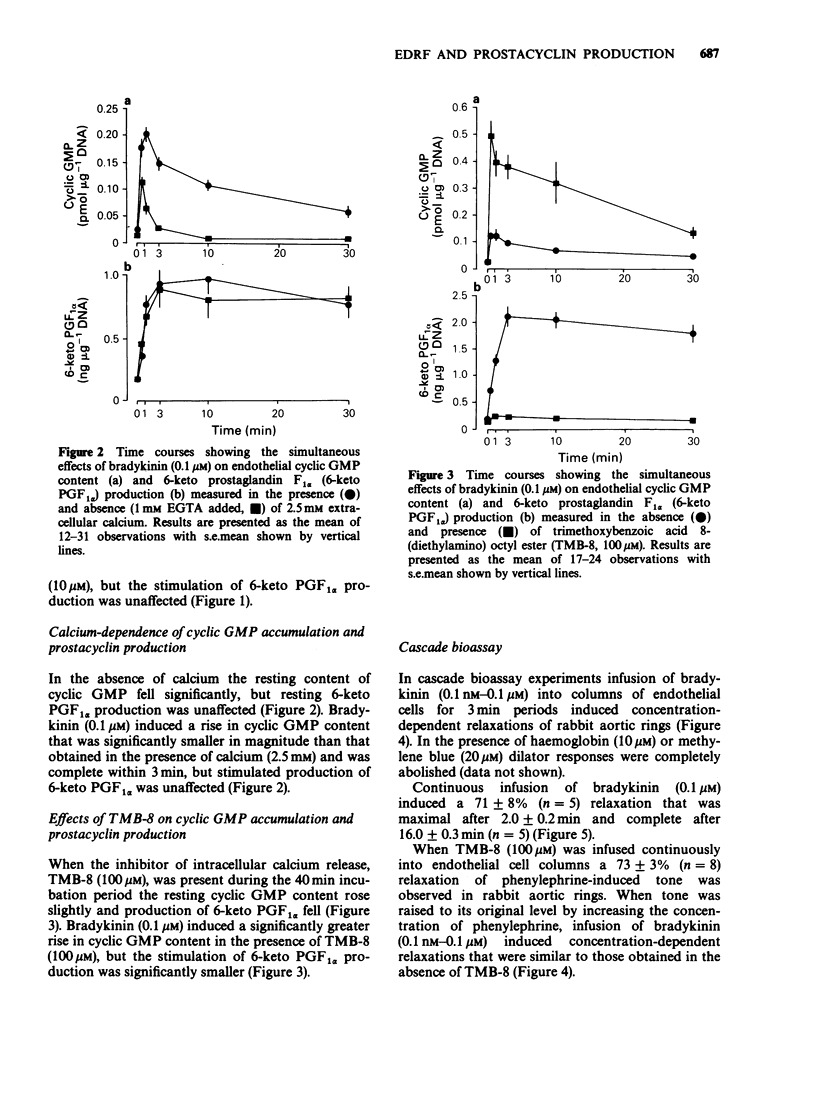
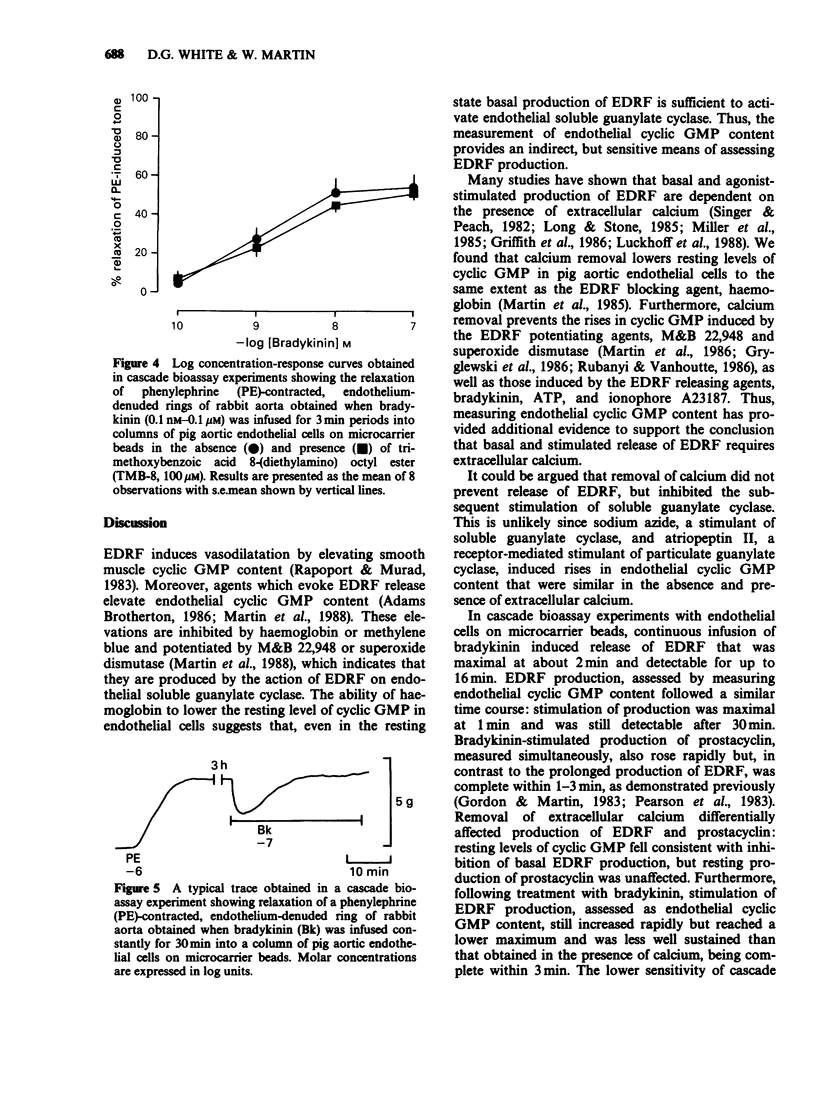
1. Production of endothelium-derived relaxing factor (EDRF) by primary cultures of pig aortic endothelial cells was assessed indirectly by measuring endothelial cyclic GMP content, and prostacyclin production was measured by radioimmunoassay of 6-keto prostaglandin F1 alpha (6-keto PGF1 alpha). 2. The resting level of cyclic GMP fell significantly following removal of extracellular calcium (1 mM EGTA present), but elevations of cyclic GMP content induced by sodium azide (10 microM) or atriopeptin II (10 nM) were similar in the absence and presence of extracellular calcium. 3. Haemoglobin (10 microM) reduced the resting level of cyclic GMP in the presence, but not the absence of extracellular calcium. M&B 22,948 (100 microM), superoxide dismutase (30 u ml-1), bradykinin (0.1 microM), ATP (10 microM) and ionophore A23187 (0.1 microM) each induced an increase in endothelial cyclic GMP content that was reduced in the absence of extracellular calcium. 4. In cascade bioassay experiments using endothelial cells on microcarrier beads and perfused in columns, continuous infusion of bradykinin (0.1 microM) induced release of EDRF, assayed on rabbit aortic rings, that was maximal after 2 min and still detectable up to about 16 min. 5. In the presence of extracellular calcium, the time course of bradykinin (0.1 microM)-stimulated production of EDRF, assessed as endothelial cyclic GMP content was maximal within 1 min, declined thereafter, but was still significant after 30 min. Production of 6-keto PGF1 alpha, measured simultaneously rose rapidly but was complete within 3 min. 6. In the absence of extracellular calcium the resting endothelial content of cyclic GMP fell, but resting production of 6-keto PGF1 alpha was unaffected.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brotherton A. F. Induction of prostacyclin biosynthesis is closely associated with increased guanosine 3',5'-cyclic monophosphate accumulation in cultured human endothelium. J Clin Invest. 1986 Nov;78(5):1253–1260. doi: 10.1172/JCI112709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colden-Stanfield M., Schilling W. P., Ritchie A. K., Eskin S. G., Navarro L. T., Kunze D. L. Bradykinin-induced increases in cytosolic calcium and ionic currents in cultured bovine aortic endothelial cells. Circ Res. 1987 Nov;61(5):632–640. doi: 10.1161/01.res.61.5.632. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Alexander R. W. Angiotensin II stimulation of prostaglandin production in cultured human vascular endothelium. Science. 1975 Jul 18;189(4198):219–220. doi: 10.1126/science.1138377. [DOI] [PubMed] [Google Scholar]
- Gordon J. L., Martin W. Stimulation of endothelial prostacyclin production plays no role in endothelium-dependent relaxation of the pig aorta. Br J Pharmacol. 1983 Sep;80(1):179–186. doi: 10.1111/j.1476-5381.1983.tb11064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski E. F., Jaffe E. A., Weksler B. B. Prostacyclin production by cultured endothelial cell monolayers exposed to step increases in shear stress. J Lab Clin Med. 1985 Jan;105(1):36–43. [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Newby A. C., Lewis M. J., Henderson A. H. Production of endothelium derived relaxant factor is dependent on oxidative phosphorylation and extracellular calcium. Cardiovasc Res. 1986 Jan;20(1):7–12. doi: 10.1093/cvr/20.1.7. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Hong S. L., Deykin D. Activation of phospholipases A2 and C in pig aortic endothelial cells synthesizing prostacyclin. J Biol Chem. 1982 Jun 25;257(12):7151–7154. [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Long C. J., Stone T. W. The release of endothelium-derived relaxant factor is calcium dependent. Blood Vessels. 1985;22(4):205–208. doi: 10.1159/000158602. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Pohl U., Mülsch A., Busse R. Differential role of extra- and intracellular calcium in the release of EDRF and prostacyclin from cultured endothelial cells. Br J Pharmacol. 1988 Sep;95(1):189–196. doi: 10.1111/j.1476-5381.1988.tb16564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Phosphodiesterase inhibitors induce endothelium-dependent relaxation of rat and rabbit aorta by potentiating the effects of spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):539–547. [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Martin W., White D. G., Henderson A. H. Endothelium-derived relaxing factor and atriopeptin II elevate cyclic GMP levels in pig aortic endothelial cells. Br J Pharmacol. 1988 Jan;93(1):229–239. doi: 10.1111/j.1476-5381.1988.tb11426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. C., Schoeffter P., Stoclet J. C. Insensitivity of calcium-dependent endothelial stimulation in rat isolated aorta to the calcium entry blocker, flunarizine. Br J Pharmacol. 1985 Jun;85(2):481–487. doi: 10.1111/j.1476-5381.1985.tb08885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Slakey L. L., Gordon J. L. Stimulation of prostaglandin production through purinoceptors on cultured porcine endothelial cells. Biochem J. 1983 Jul 15;214(1):273–276. doi: 10.1042/bj2140273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Seid J. M., MacNeil S., Tomlinson S. Calcium, calmodulin, and the production of prostacyclin by cultured vascular endothelial cells. Biosci Rep. 1983 Nov;3(11):1007–1015. doi: 10.1007/BF01121027. [DOI] [PubMed] [Google Scholar]
- Singer H. A., Peach M. J. Calcium- and endothelial-mediated vascular smooth muscle relaxation in rabbit aorta. Hypertension. 1982 May-Jun;4(3 Pt 2):19–25. [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]


