Abstract
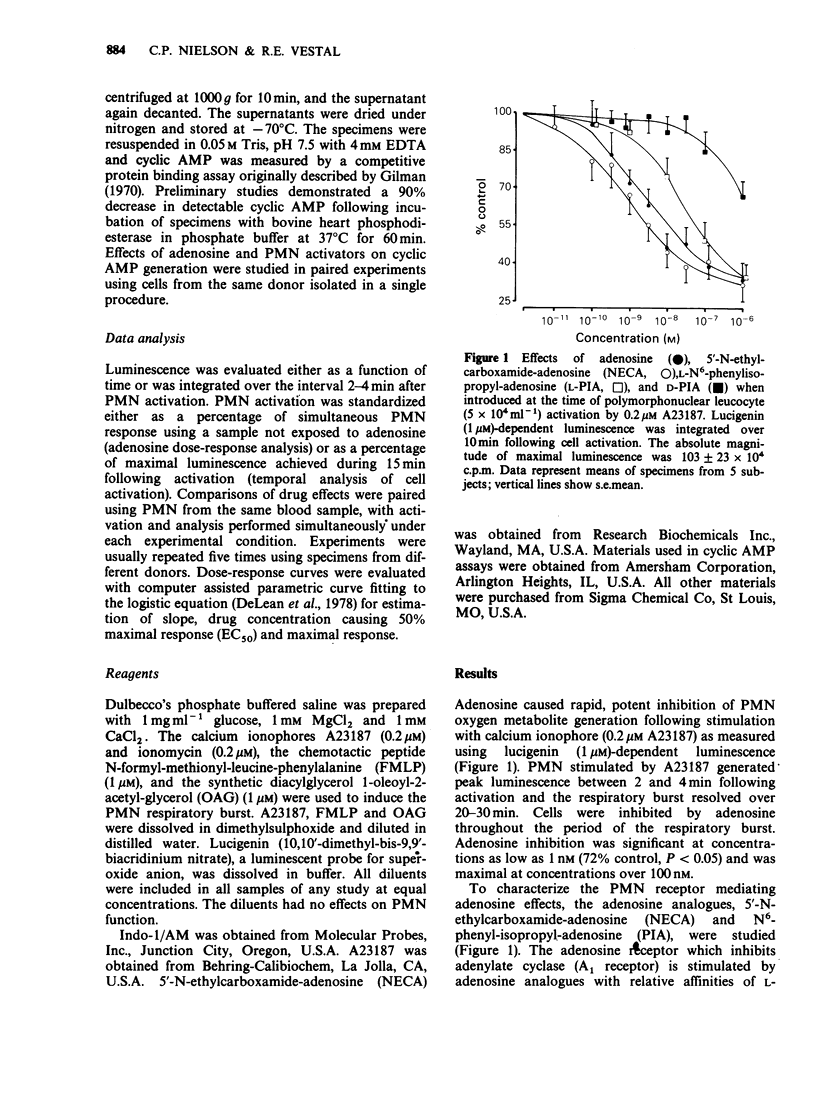
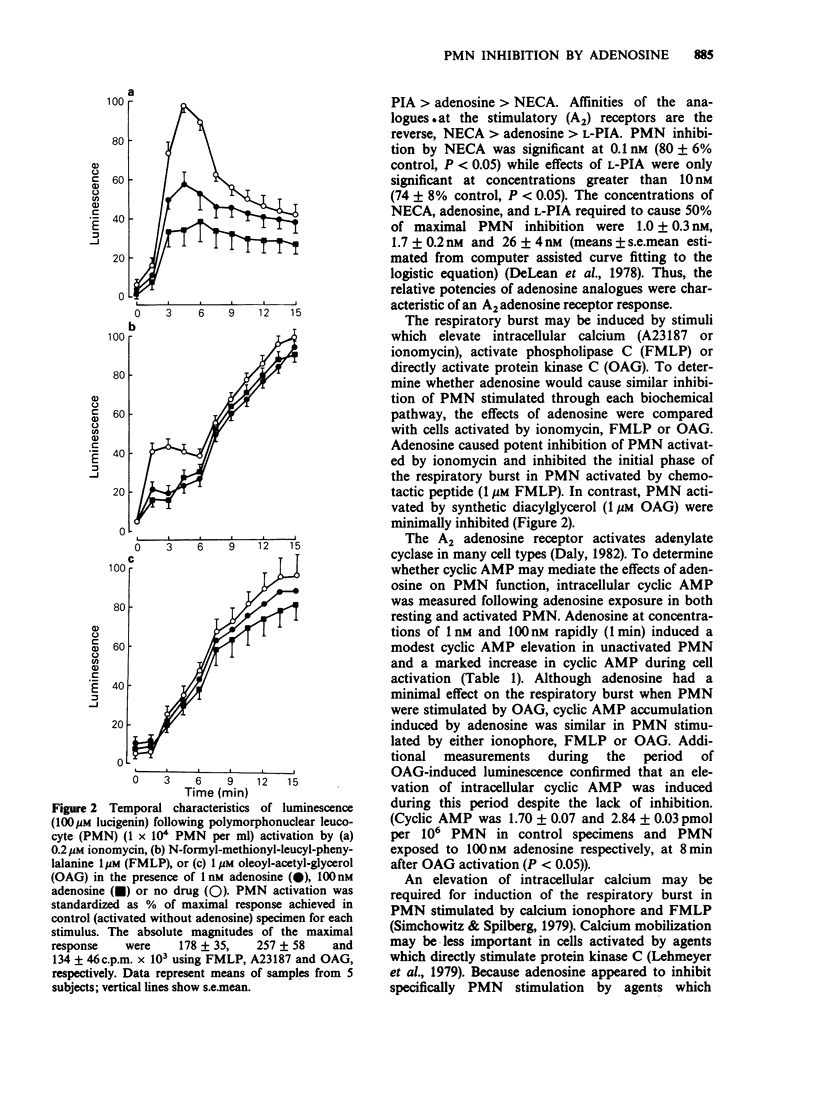
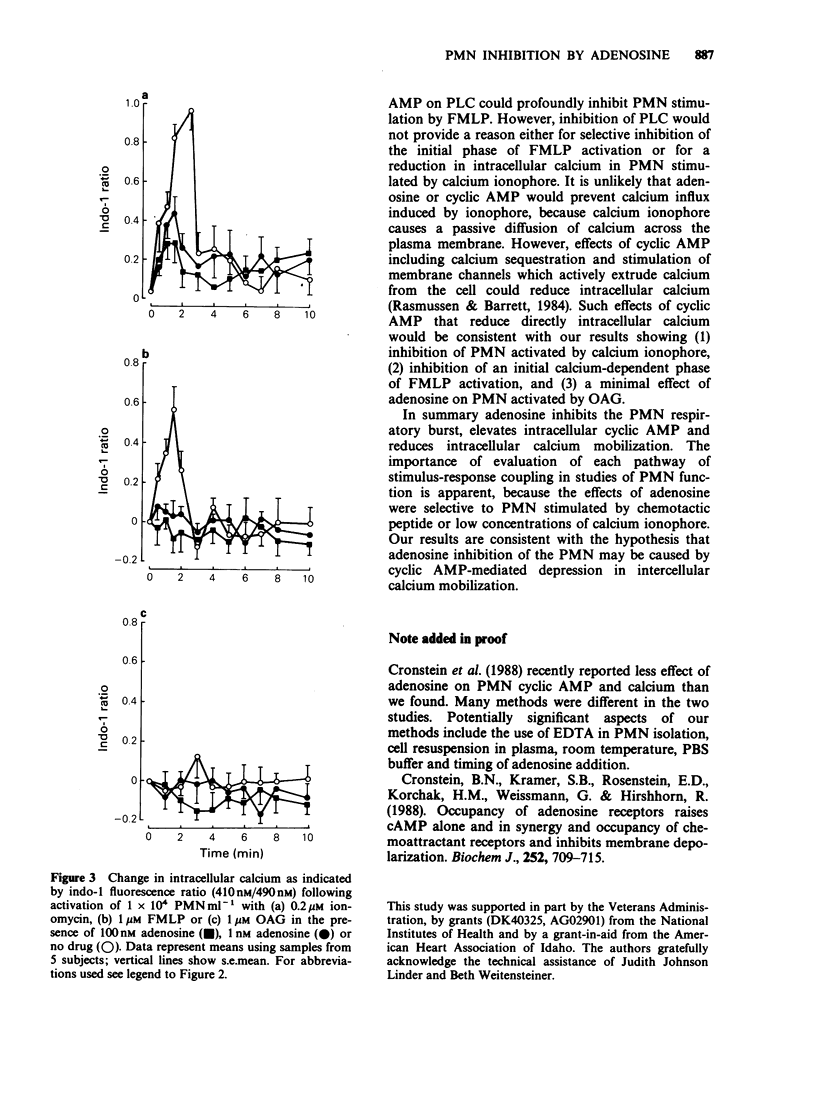
1. Inhibition of human polymorphonuclear leucocyte (PMN) function by adenosine was studied with respect to effects of adenosine on intracellular cyclic AMP and calcium during the PMN respiratory burst. 2. The adenosine analogue 5'-N-ethylcarboxamide-adenosine (NECA) and L-N6-phenyl-isopropyl-adenosine (L-PIA) inhibited PMN oxygen metabolite generation with relative potencies (NECA greater than adenosine greater than L-PIA) characteristic of an A2 receptor. 3. The respiratory burst was inhibited by adenosine when PMN were activated by calcium ionophore or chemotactic peptide but not when cells where activated by oleoyl-acetyl-glycerol (OAG). 4. Adenosine increased intracellular cyclic AMP during the PMN respiratory burst regardless of whether cells were stimulated by ionophore, chemotactic peptide or OAG. 5. To determine whether the differences in cell inhibition by adenosine were related to differences in intracellular calcium mobilization by each activating agent, calcium was evaluated with the fluorescent probe, indo-1. Adenosine suppressed the increase in intracellular calcium following PMN activation by calcium ionophore or chemotactic peptide. In contrast, calcium did not increase in PMN activated by OAG and adenosine did not affect intracellular calcium changes following this stimulus. 6. These results demonstrate that physiological concentrations of adenosine inhibit the PMN respiratory burst in association with an increase in intracellular cyclic AMP and reduction of intracellular calcium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse W. W., Sosman J. M. Isoproterenol inhibition of isolated human neutrophil function. J Allergy Clin Immunol. 1984 Mar;73(3):404–410. doi: 10.1016/0091-6749(84)90416-0. [DOI] [PubMed] [Google Scholar]
- Chagoya de Sánchez V., Hernández-Muñoz R., Díaz-Muñoz M., Villalobos R., Glender W., Vidrio S., Suárez J., Yañez L. Circadian variations of adenosine level in blood and liver and its possible physiological significance. Life Sci. 1983 Sep 12;33(11):1057–1064. doi: 10.1016/0024-3205(83)90661-6. [DOI] [PubMed] [Google Scholar]
- Chari-Bitron A., Motola L., Kaplun A., Shahar A. Correlation between chemiluminescence response and rate of zymosan uptake by rat alveolar macrophages. Scanning electron microscope observations. Cell Biol Int Rep. 1983 Apr;7(4):303–310. doi: 10.1016/0309-1651(83)90065-6. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Rosenstein E. D., Korchak H. M., Weissmann G., Hirschhorn R. Occupancy of adenosine receptors raises cyclic AMP alone and in synergy with occupancy of chemoattractant receptors and inhibits membrane depolarization. Biochem J. 1988 Jun 15;252(3):709–715. doi: 10.1042/bj2520709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med. 1983 Oct 1;158(4):1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Kubersky S. M., Weissmann G., Hirschhorn R. Engagement of adenosine receptors inhibits hydrogen peroxide (H2O2-) release by activated human neutrophils. Clin Immunol Immunopathol. 1987 Jan;42(1):76–85. doi: 10.1016/0090-1229(87)90174-7. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Rosenstein E. D., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol. 1985 Aug;135(2):1366–1371. [PubMed] [Google Scholar]
- Dahinden C. A., Fehr J., Hugli T. E. Role of cell surface contact in the kinetics of superoxide production by granulocytes. J Clin Invest. 1983 Jul;72(1):113–121. doi: 10.1172/JCI110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. W. Adenosine receptors: targets for future drugs. J Med Chem. 1982 Mar;25(3):197–207. doi: 10.1021/jm00345a001. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Della Bianca V., De Togni P., Grzeskowiak M., Vicentini L. M., Di Virgilio F. Cyclic AMP inhibition of phosphoinositide turnover in human neutrophils. Biochim Biophys Acta. 1986 May 29;886(3):441–447. doi: 10.1016/0167-4889(86)90180-1. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Ignarro L. J., Colombo C. Enzyme release from polymorphonuclear leukocyte lysosomes: regulation by autonomic drugs and cyclic nucleotides. Science. 1973 Jun 15;180(4091):1181–1183. doi: 10.1126/science.180.4091.1181. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- Lehmeyer J. E., Snyderman R., Johnston R. B., Jr Stimulation of neutrophil oxidative metabolism by chemotactic peptides: influence of calcium ion concentration and cytochalasin B and comparison with stimulation by phorbol myristate acetate. Blood. 1979 Jul;54(1):35–45. [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack J. A., Nielson C. P., Stevens D. L., Vestal R. E. Beta-adrenoceptor-mediated modulation of calcium ionophore activated polymorphonuclear leucocytes. Br J Pharmacol. 1986 Jun;88(2):417–423. doi: 10.1111/j.1476-5381.1986.tb10219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marone G., Petracca R., Vigorita S. Adenosine receptors on human inflammatory cells. Int Arch Allergy Appl Immunol. 1985;77(1-2):259–263. doi: 10.1159/000233805. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Snyderman R. Activation of the respiratory burst enzyme in human polymorphonuclear leukocytes by chemoattractants and other soluble stimuli. Evidence that the same oxidase is activated by different transductional mechanisms. J Clin Invest. 1983 Jul;72(1):192–200. doi: 10.1172/JCI110957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson C. P. Beta-adrenergic modulation of the polymorphonuclear leukocyte respiratory burst is dependent upon the mechanism of cell activation. J Immunol. 1987 Oct 1;139(7):2392–2397. [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. The calcium messenger system (1). N Engl J Med. 1986 Apr 24;314(17):1094–1101. doi: 10.1056/NEJM198604243141707. [DOI] [PubMed] [Google Scholar]
- Schrier D. J., Imre K. M. The effects of adenosine agonists on human neutrophil function. J Immunol. 1986 Nov 15;137(10):3284–3289. [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Generation of superoxide radicals by human peripheral neutrophils activated by chemotactic factor. Evidence for the role of calcium. J Lab Clin Med. 1979 Apr;93(4):583–593. [PubMed] [Google Scholar]
- Takenawa T., Homma Y., Nagai Y. Role of Ca2+ in phosphatidylinositol response and arachidonic acid release in formylated tripeptide- or Ca2+ ionophore A23187-stimulated guinea pig neutrophils. J Immunol. 1983 Jun;130(6):2849–2855. [PubMed] [Google Scholar]
- Takenawa T., Ishitoya J., Homma Y., Kato M., Nagai Y. Role of enhanced inositol phospholipid metabolism in neutrophil activation. Biochem Pharmacol. 1985 Jun 1;34(11):1931–1935. doi: 10.1016/0006-2952(85)90311-9. [DOI] [PubMed] [Google Scholar]
- Wolff J., Londos C., Cooper D. M. Adenosine receptors and the regulation of adenylate cyclase. Adv Cyclic Nucleotide Res. 1981;14:199–214. [PubMed] [Google Scholar]


