Abstract
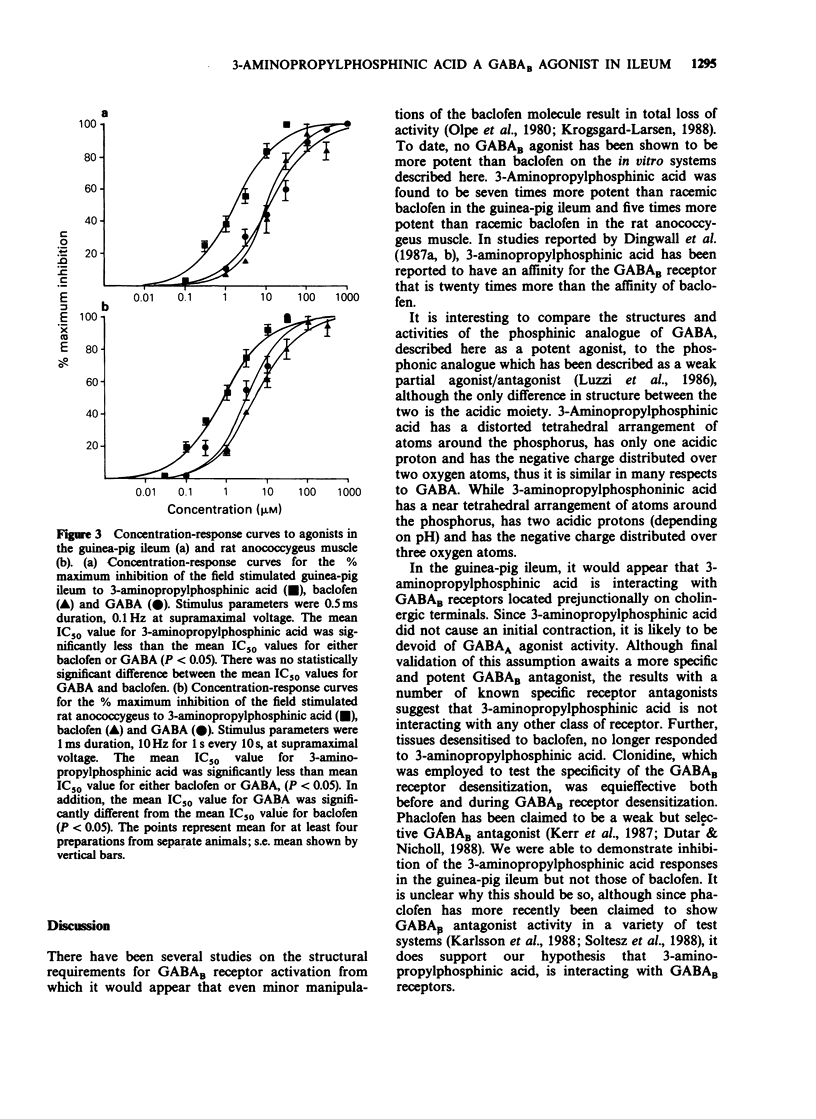
1. 3-Aminopropylphosphinic acid, a gamma-aminobutyric acid (GABA) analogue, was tested for activity on guinea-pig isolated ileum and rat isolated anococcygeus muscle preparations. The effects of 3-aminopropylphosphinic acid were compared with those of GABA and baclofen. 2. In the electrically stimulated ileum, 3-aminopropylphosphinic acid, like GABA and baclofen, caused a concentration-dependent inhibition of the cholinergic twitch contraction, the IC50 value being 1.84 +/- 0.23 microM (n = 12). Unlike GABA, but like baclofen, 3-aminopropylphosphinic acid did not produce an initial contraction. 3. The inhibitory effects of 3-aminopropylphosphinic acid and baclofen in the guinea-pig ileum were not significantly antagonized by bicuculline (10 microM), phentolamine plus propranolol (both 1 microM), yohimbine (1 microM), naloxone (1 microM), impromidine (1 microM) or 8-phenyltheophylline (10 microM). The inhibitory effects of 3-aminopropylphosphinic acid, but not of baclofen, were however antagonized by phaclofen (500 microM). In addition the effects of 3-aminopropylphosphinic acid were abolished by baclofen desensitization in the guinea-pig ileum. 4. 3-Aminopropylphosphinic acid, GABA and baclofen reduced the twitch contraction evoked by electrical field stimulation in the rat anococcygeus muscle. The IC50 for 3-aminopropylphosphinic acid inhibition of the anococcygeus contraction was 0.89 +/- 0.15 microM (n = 8). 5. It is concluded that 3-aminopropylphosphinic acid is a potent, selective GABAB agonist, being seven times more potent than baclofen in the guinea-pig ileum and five times more potent than baclofen in the rat anococcygues muscle preparations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULBRING E. Measurements of oxygen consumption in smooth muscle. J Physiol. 1953 Oct;122(1):111–134. doi: 10.1113/jphysiol.1953.sp004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Doble A., Hill D. R., Hudson A. L., Shaw J. S., Turnbull M. J., Warrington R. Bicuculline-insensitive GABA receptors on peripheral autonomic nerve terminals. Eur J Pharmacol. 1981 Apr 24;71(1):53–70. doi: 10.1016/0014-2999(81)90386-1. [DOI] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. A physiological role for GABAB receptors in the central nervous system. Nature. 1988 Mar 10;332(6160):156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S. The rat anococcygeus muscle and its response to nerve stimulation and to some drugs. Br J Pharmacol. 1972 Jul;45(3):404–416. doi: 10.1111/j.1476-5381.1972.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giotti A., Luzzi S., Spagnesi S., Zilletti L. GABAA and GABAB receptor-mediated effects in guinea-pig ileum. Br J Pharmacol. 1983 Mar;78(3):469–478. doi: 10.1111/j.1476-5381.1983.tb08807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplita P. V., Waters D. H., Triggle D. J. gamma-Aminobutyric acid action in guinea-pig ileal myenteric plexus. Eur J Pharmacol. 1982 Apr 8;79(1-2):43–51. doi: 10.1016/0014-2999(82)90573-8. [DOI] [PubMed] [Google Scholar]
- Karlsson G., Pozza M., Olpe H. R. Phaclofen: a GABAB blocker reduces long-duration inhibition in the neocortex. Eur J Pharmacol. 1988 Apr 13;148(3):485–486. doi: 10.1016/0014-2999(88)90136-7. [DOI] [PubMed] [Google Scholar]
- Kerr D. I., Ong J., Prager R. H., Gynther B. D., Curtis D. R. Phaclofen: a peripheral and central baclofen antagonist. Brain Res. 1987 Mar 3;405(1):150–154. doi: 10.1016/0006-8993(87)90999-1. [DOI] [PubMed] [Google Scholar]
- Krantis A., Costa M., Furness J. B., Orbach J. gamma-Aminobutyric acid stimulates intrinsic inhibitory and excitatory nerves in the guinea-pig intestine. Eur J Pharmacol. 1980 Oct 31;67(4):461–468. doi: 10.1016/0014-2999(80)90187-9. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P. GABA synaptic mechanisms: stereochemical and conformational requirements. Med Res Rev. 1988 Jan-Mar;8(1):27–56. doi: 10.1002/med.2610080103. [DOI] [PubMed] [Google Scholar]
- Luzzi S., Franchi-Micheli S., Ciuffi M., Pajani A., Zilletti L. GABA-related activities of amino phosphonic acids on guinea-pig ileum longitudinal muscle. J Auton Pharmacol. 1986 Sep;6(3):163–169. doi: 10.1111/j.1474-8673.1986.tb00641.x. [DOI] [PubMed] [Google Scholar]
- Muhyaddin M., Roberts P. J., Woodruff G. N. Presynaptic gamma-aminobutyric acid receptors in the rat anococcygeus muscle and their antagonism by 5-aminovaleric acid. Br J Pharmacol. 1982 Sep;77(1):163–168. doi: 10.1111/j.1476-5381.1982.tb09282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong J., Kerr D. I. GABAA- and GABAB-receptor-mediated modification of intestinal motility. Eur J Pharmacol. 1982 Dec 17;86(1):9–17. doi: 10.1016/0014-2999(82)90390-9. [DOI] [PubMed] [Google Scholar]
- Soltesz I., Haby M., Leresche N., Crunelli V. The GABAB antagonist phaclofen inhibits the late K+-dependent IPSP in cat and rat thalamic and hippocampal neurones. Brain Res. 1988 May 17;448(2):351–354. doi: 10.1016/0006-8993(88)91275-9. [DOI] [PubMed] [Google Scholar]


