Abstract
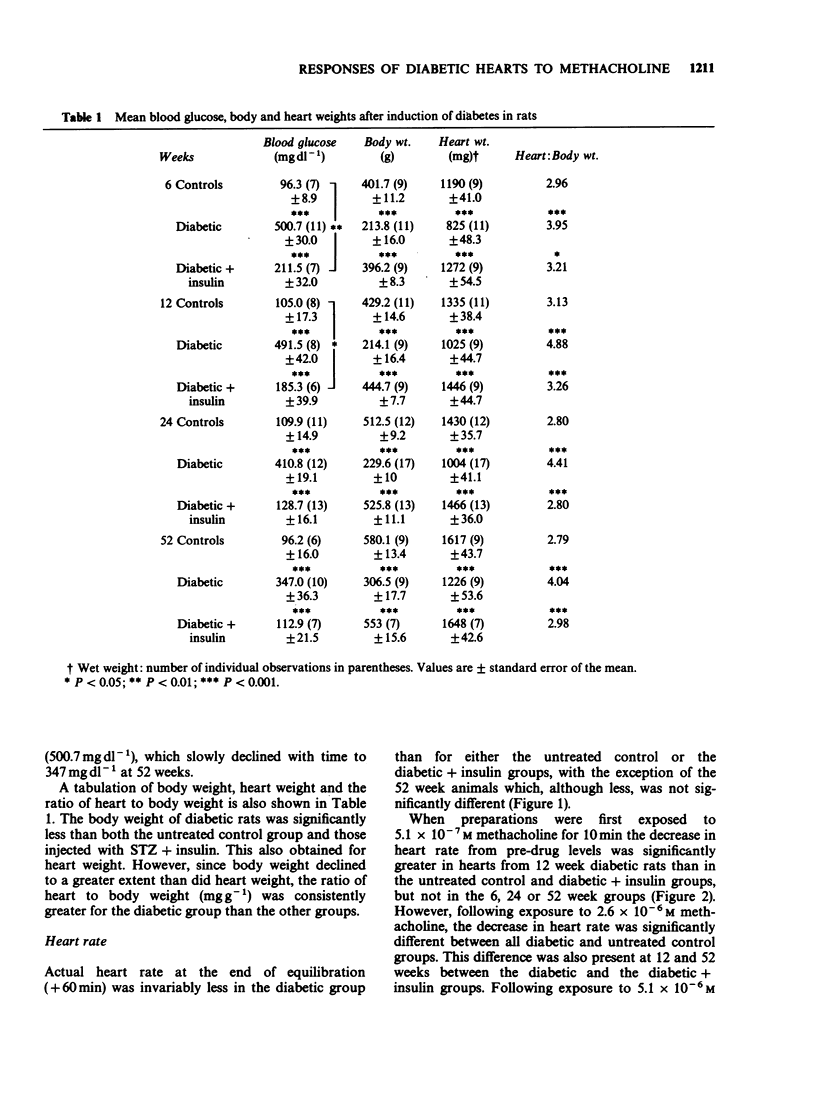
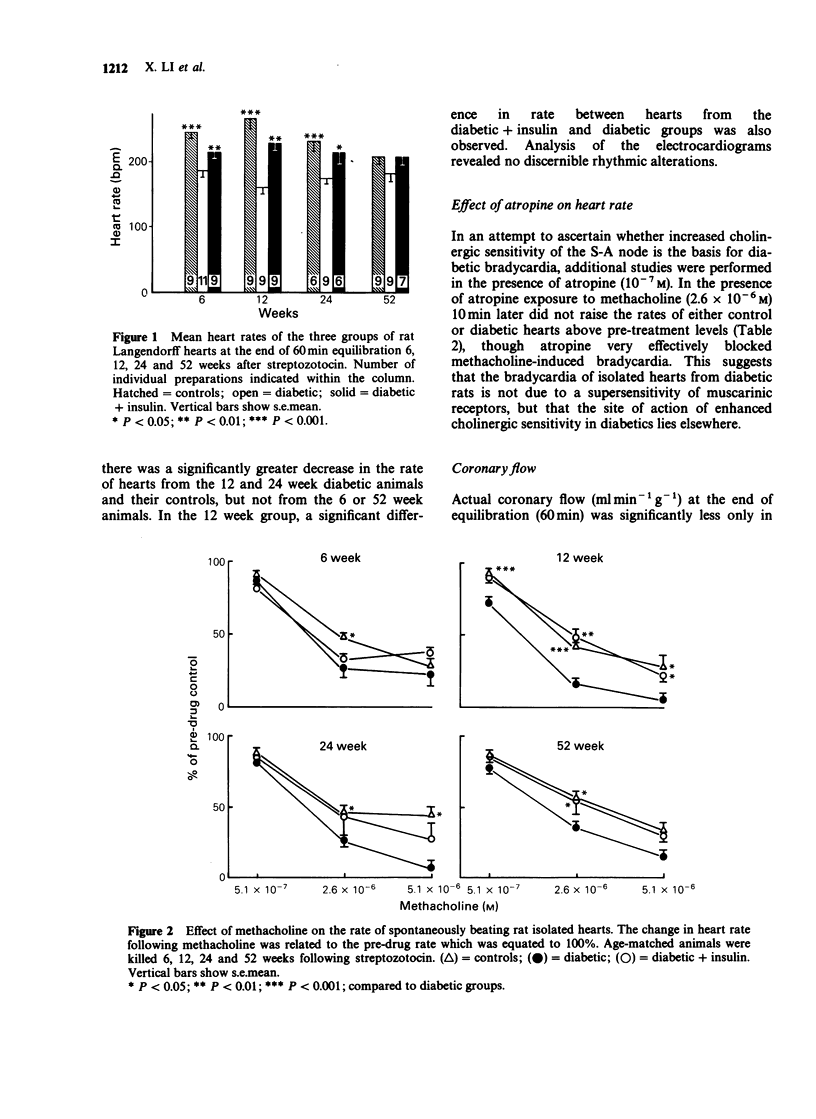
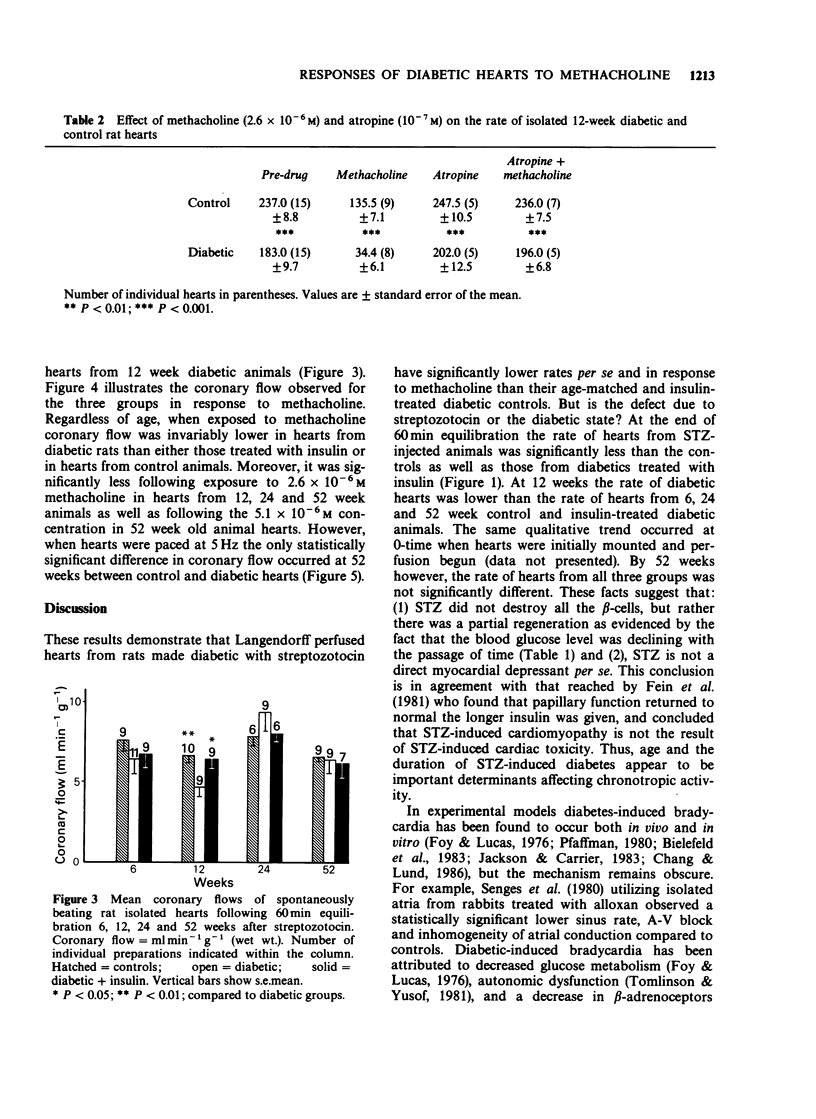
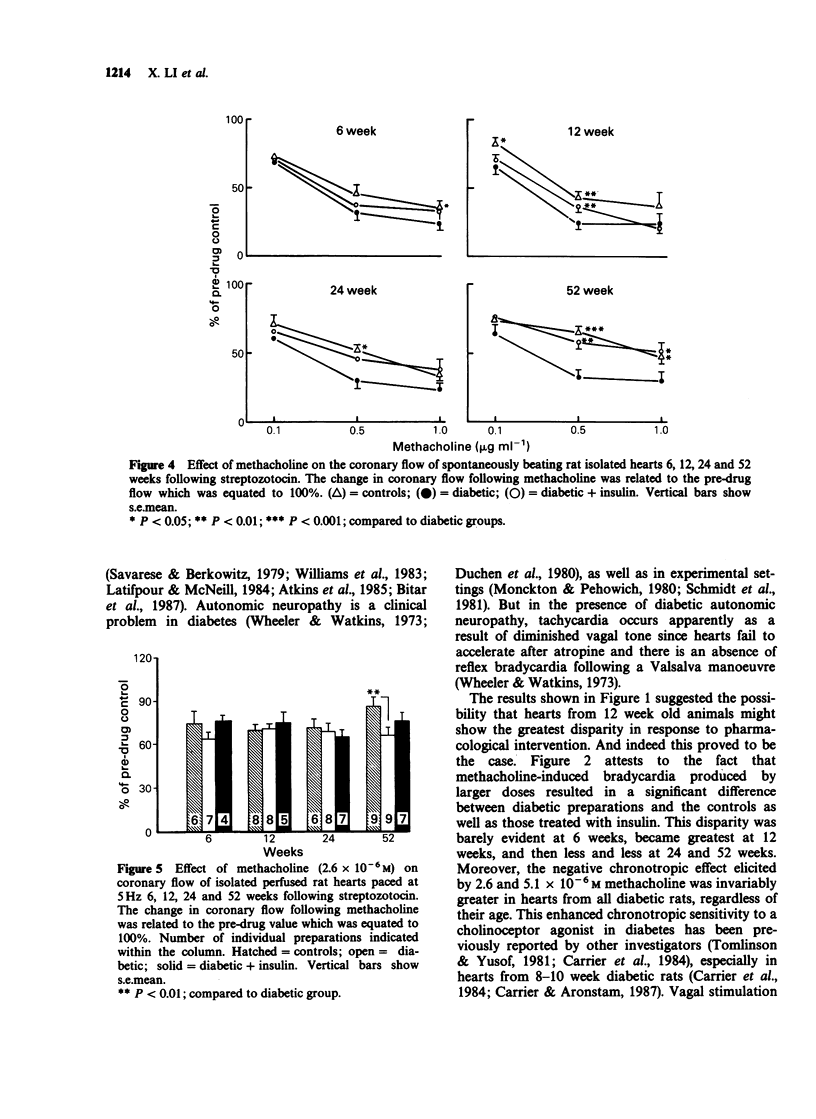
1. Isolated hearts perfused by the method of Langendorff from 6, 12 and 24 week streptozotocin (STZ) diabetic rats displayed a significant bradycardia following 60 min equilibration. The rate of hearts from 12-week diabetic rats (164 +/- 17) displayed the greatest bradycardia compared to age-matched controls (268 +/- 15; P less than 0.001), and diabetics treated with insulin (232 +/- 17; P less than 0.01), but by 52 weeks the heart rate of the 3 groups was similar. With advancing age the effect of STZ diabetes on the rate of rat isolated perfused hearts remained unchanged but the rate of the control and diabetic + insulin groups declined. 2. Hearts from 6-52 week STZ-treated rats were found to be more sensitive to the negative chronotropic effect of methacholine, the greatest difference occurring in hearts from the 12 week animals. Atropine (10(-7) M) did not affect the resting heart rate of age-matched controls or diabetics but blocked methacholine (2.6 x 10(-6) M)-induced bradycardia in both, suggesting that the site of action of diabetic bradycardia is not the muscarinic receptors. 3. At the end of equilibration there was a significant decrease in coronary flow in hearts from 12 week diabetic animals. In spontaneously beating diabetic rat hearts administration of methacholine (2.6 x 10(-6) M) produced a significantly greater decrease in coronary flow in the 12, 24 and 52 week diabetic hearts. When electrically paced (5 Hz) however, there was no difference in response to methacholine between the three groups except at 52 weeks between the age-matched control and diabetic groups.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins F. L., Dowell R. T., Love S. beta-Adrenergic receptors, adenylate cyclase activity, and cardiac dysfunction in the diabetic rat. J Cardiovasc Pharmacol. 1985 Jan-Feb;7(1):66–70. doi: 10.1097/00005344-198501000-00011. [DOI] [PubMed] [Google Scholar]
- BERNE R. M. REGULATION OF CORONARY BLOOD FLOW. Physiol Rev. 1964 Jan;44:1–29. doi: 10.1152/physrev.1964.44.1.1. [DOI] [PubMed] [Google Scholar]
- Bielefeld D. R., Pace C. S., Boshell B. R. Altered sensitivity of chronic diabetic rat heart to calcium. Am J Physiol. 1983 Dec;245(6):E560–E567. doi: 10.1152/ajpendo.1983.245.6.E560. [DOI] [PubMed] [Google Scholar]
- Bitar M. S., Koulu M., Rapoport S. I., Linnoila M. Adrenal catecholamine metabolism and myocardial adrenergic receptors in streptozotocin diabetic rats. Biochem Pharmacol. 1987 Apr 1;36(7):1011–1016. doi: 10.1016/0006-2952(87)90407-2. [DOI] [PubMed] [Google Scholar]
- Blumenthal M. R., Wang H. H., Markee S., Wang S. C. Effects of acetylcholine on the heart. Am J Physiol. 1968 Jun;214(6):1280–1287. doi: 10.1152/ajplegacy.1968.214.6.1280. [DOI] [PubMed] [Google Scholar]
- Carrier G. O., Aronstam R. S. Altered muscarinic receptor properties and function in the heart in diabetes. J Pharmacol Exp Ther. 1987 Aug;242(2):531–535. [PubMed] [Google Scholar]
- Carrier G. O., Edwards A. D., Aronstam R. S. Cholinergic supersensitivity and decreased number of muscarinic receptors in atria from short-term diabetic rats. J Mol Cell Cardiol. 1984 Oct;16(10):963–965. doi: 10.1016/s0022-2828(84)80032-2. [DOI] [PubMed] [Google Scholar]
- Cavaliere T. A., Taylor D. G., Kerwin L. J., Antonaccio M. J. Cardiovascular effects of alloxan diabetes in normotensive and spontaneously hypertensive rats. Pharmacology. 1980;20(4):211–223. doi: 10.1159/000137367. [DOI] [PubMed] [Google Scholar]
- Chang K. S., Lund D. D. Alterations in the baroreceptor reflex control of heart rate in streptozotocin diabetic rats. J Mol Cell Cardiol. 1986 Jun;18(6):617–624. doi: 10.1016/s0022-2828(86)80969-5. [DOI] [PubMed] [Google Scholar]
- Clarke B. F., Ewing D. J., Campbell I. W. Diabetic autonomic neuropathy. Diabetologia. 1979 Oct;17(4):195–212. doi: 10.1007/BF01235856. [DOI] [PubMed] [Google Scholar]
- Dhalla N. S., Pierce G. N., Innes I. R., Beamish R. E. Pathogenesis of cardiac dysfunction in diabetes mellitus. Can J Cardiol. 1985 Jul-Aug;1(4):263–281. [PubMed] [Google Scholar]
- Duchen L. W., Anjorin A., Watkins P. J., Mackay J. D. Pathology of autonomic neuropathy in diabetes mellitus. Ann Intern Med. 1980 Feb;92(2 Pt 2):301–303. doi: 10.7326/0003-4819-92-2-301. [DOI] [PubMed] [Google Scholar]
- Elfellah M. S., Johns A., Shepherd A. M. Effect of age on responsiveness of isolated rat atria to carbachol and on binding characteristics of atrial muscarinic receptors. J Cardiovasc Pharmacol. 1986 Jul-Aug;8(4):873–877. doi: 10.1097/00005344-198709010-00032. [DOI] [PubMed] [Google Scholar]
- Fein F. S., Strobeck J. E., Malhotra A., Scheuer J., Sonnenblick E. H. Reversibility of diabetic cardiomyopathy with insulin in rats. Circ Res. 1981 Dec;49(6):1251–1261. doi: 10.1161/01.res.49.6.1251. [DOI] [PubMed] [Google Scholar]
- Foy J. M., Lucas P. D. Effect of experimental diabetes, food deprivation and genetic obesity on the sensitivity of pithed rats to autonomic agents. Br J Pharmacol. 1976 Jun;57(2):229–234. doi: 10.1111/j.1476-5381.1976.tb07472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg R. The isolated human epicardial coronary artery. Am J Cardiol. 1983 Jul 20;52(2):61A–66A. doi: 10.1016/0002-9149(83)90178-9. [DOI] [PubMed] [Google Scholar]
- Hamby R. I., Zoneraich S., Sherman L. Diabetic cardiomyopathy. JAMA. 1974 Sep 23;229(13):1749–1754. [PubMed] [Google Scholar]
- Jackson C. V., Carrier G. O. Influence of short-term experimental diabetes on blood pressure and heart rate in response to norepinephrine and angiotensin II in the conscious rat. J Cardiovasc Pharmacol. 1983 Mar-Apr;5(2):260–265. doi: 10.1097/00005344-198303000-00016. [DOI] [PubMed] [Google Scholar]
- Kalsner S. Cholinergic mechanisms in human coronary artery preparations: implications of species differences. J Physiol. 1985 Jan;358:509–526. doi: 10.1113/jphysiol.1985.sp015564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel W. B., Hjortland M., Castelli W. P. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974 Jul;34(1):29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- Latifpour J., McNeill J. H. Cardiac autonomic receptors: effect of long-term experimental diabetes. J Pharmacol Exp Ther. 1984 Jul;230(1):242–249. [PubMed] [Google Scholar]
- Ledet T. Diabetic cardiopathy. Quantitative histological studies of the heart from young juvenile diabetics. Acta Pathol Microbiol Scand A. 1976 Sep;84(5):421–428. [PubMed] [Google Scholar]
- Monckton G., Pehowich E. Autonomic neuropathy in the streptozotocin diabetic rat. Can J Neurol Sci. 1980 May;7(2):135–142. doi: 10.1017/s0317167100023519. [DOI] [PubMed] [Google Scholar]
- Mueller S. M. Insulin treatment prevents vascular dysfunction in early juvenile alloxan-induced diabetes mellitus. Am J Physiol. 1984 Jul;247(1 Pt 2):H132–H138. doi: 10.1152/ajpheart.1984.247.1.H132. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Fleckenstein A., Byon Y. K., Fleckenstein-Grün G. Fundamental physiology of coronary smooth musculature from extramural stem arteries of pigs and rabbits. (Electric excitability, tension development, influence of Ca, Mg, H and K ions). Eur J Cardiol. 1978 Oct;8(3):319–335. [PubMed] [Google Scholar]
- Nuutinen E. M., Wilson D. F., Erecińska M. The effect of cholinergic agonists on coronary flow rate and oxygen consumption in isolated perfused rat heart. J Mol Cell Cardiol. 1985 Jan;17(1):31–42. doi: 10.1016/s0022-2828(85)80090-0. [DOI] [PubMed] [Google Scholar]
- Pfaffman M. A. The effects of streptozotocin-induced diabetes and insulin-treatment on the cardiovascular system of the rat. Res Commun Chem Pathol Pharmacol. 1980 Apr;28(1):27–41. [PubMed] [Google Scholar]
- Regan T. J., Lyons M. M., Ahmed S. S., Levinson G. E., Oldewurtel H. A., Ahmad M. R., Haider B. Evidence for cardiomyopathy in familial diabetes mellitus. J Clin Invest. 1977 Oct;60(4):884–899. doi: 10.1172/JCI108843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerup C. C. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev. 1970 Dec;22(4):485–518. [PubMed] [Google Scholar]
- Sakai K. Coronary vasoconstriction by locally administered acetylcholine, carbachol and bethanechol in isolated, donor-perfused, rat hearts. Br J Pharmacol. 1980 Apr;68(4):625–632. doi: 10.1111/j.1476-5381.1980.tb10853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson J. E., Brown D. J., Rivellese A., Kohner E. Diabetic cardiomyopathy? An echocardiographic study of young diabetics. Br Med J. 1978 Feb 18;1(6110):404–407. doi: 10.1136/bmj.1.6110.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarese J. J., Berkowitz B. A. beta-Adrenergic receptor decrease in diabetic rat hearts. Life Sci. 1979 Dec 10;25(24-25):2075–2078. doi: 10.1016/0024-3205(79)90200-5. [DOI] [PubMed] [Google Scholar]
- Schmidt R. E., Nelson J. S., Johnson E. M., Jr Experimental diabetic autonomic neuropathy. Am J Pathol. 1981 May;103(2):210–225. [PMC free article] [PubMed] [Google Scholar]
- Senges J., Brachmann J., Pelzer D., Hasslacher C., Weihe E., Kübler W. Altered cardiac automaticity and conduction in experimental diabetes mellitus. J Mol Cell Cardiol. 1980 Dec;12(12):1341–1351. doi: 10.1016/0022-2828(80)90120-0. [DOI] [PubMed] [Google Scholar]
- Stuesse S. L., Wallick D. W., Mace S. Vagal control of heart period in alloxan diabetic rats. Life Sci. 1982 Jul 26;31(4):393–398. doi: 10.1016/0024-3205(82)90420-9. [DOI] [PubMed] [Google Scholar]
- Tanz R. D., Russell N. J., Banerian S. P., Sharp V. H. Ouabain-induced tachyarrhythmias and cell damage in isolated perfused guinea-pig hearts: I. Protection by propranolol. J Mol Cell Cardiol. 1982 Nov;14(11):655–671. doi: 10.1016/0022-2828(82)90163-8. [DOI] [PubMed] [Google Scholar]
- Vadlamudi R. V., McNeill J. H. Effect of alloxan- and streptozotocin-induced diabetes on isolated rat heart responsiveness to carbachol. J Pharmacol Exp Ther. 1983 May;225(2):410–415. [PubMed] [Google Scholar]
- Wheeler T., Watkins P. J. Cardiac denervation in diabetes. Br Med J. 1973 Dec 8;4(5892):584–586. doi: 10.1136/bmj.4.5892.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. S., Schaible T. F., Scheuer J., Kennedy R. Effects of experimental diabetes on adrenergic and cholinergic receptors of rat myocardium. Diabetes. 1983 Oct;32(10):881–886. doi: 10.2337/diab.32.10.881. [DOI] [PubMed] [Google Scholar]


