Abstract
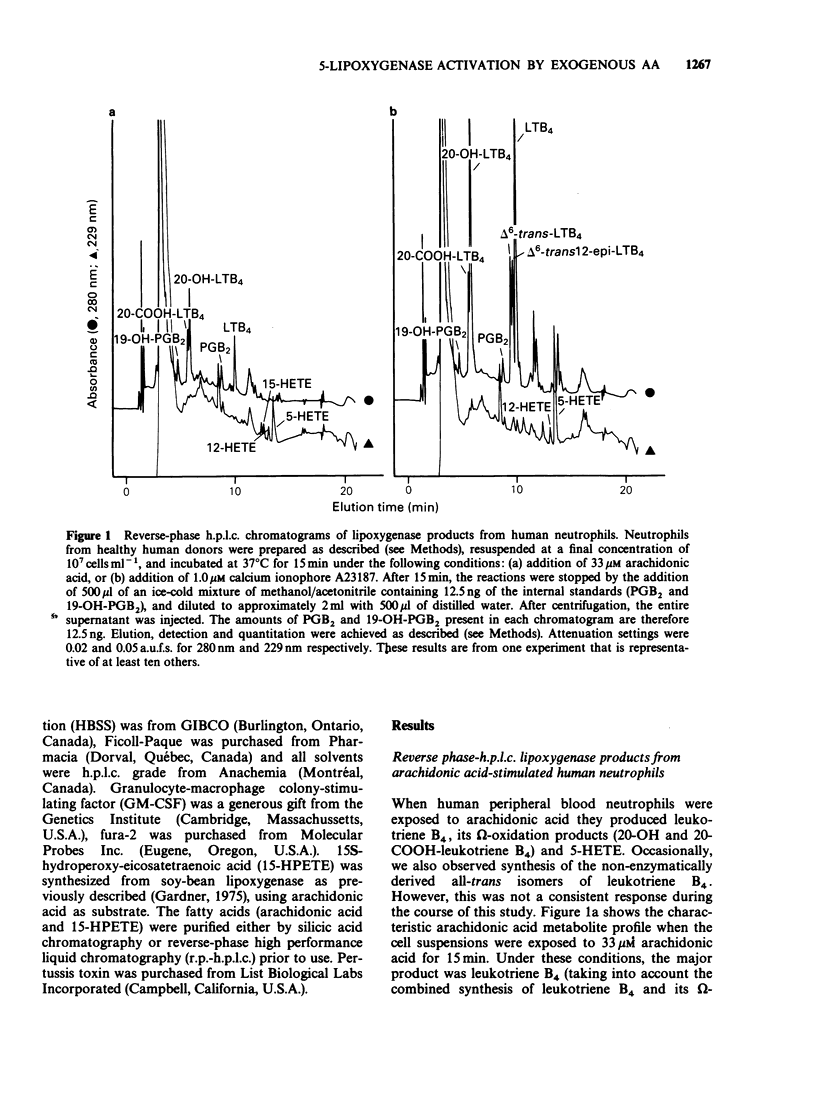
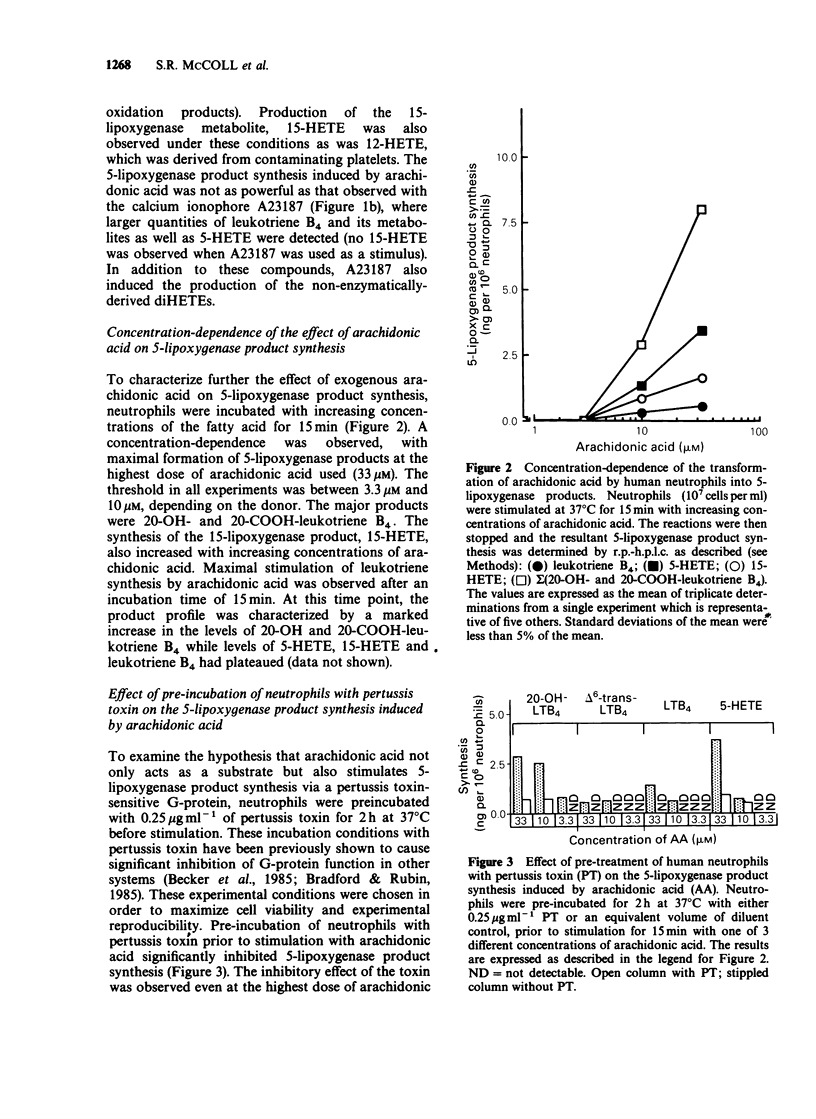
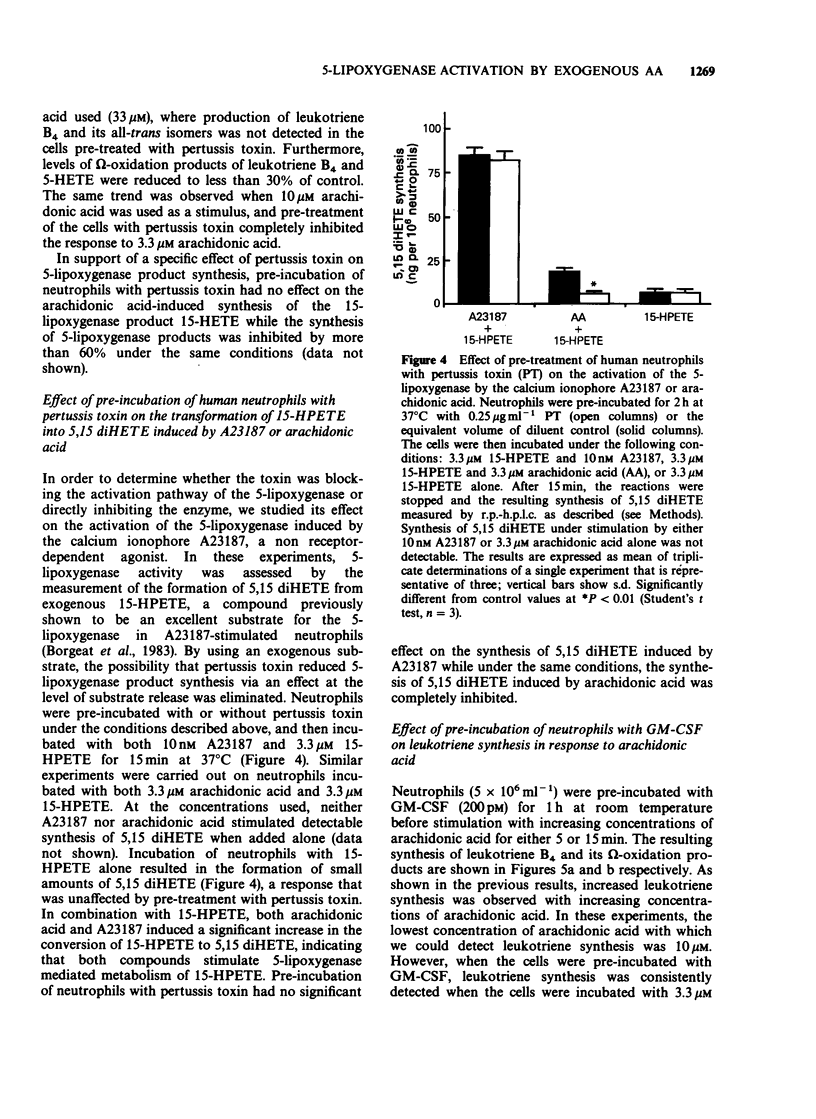
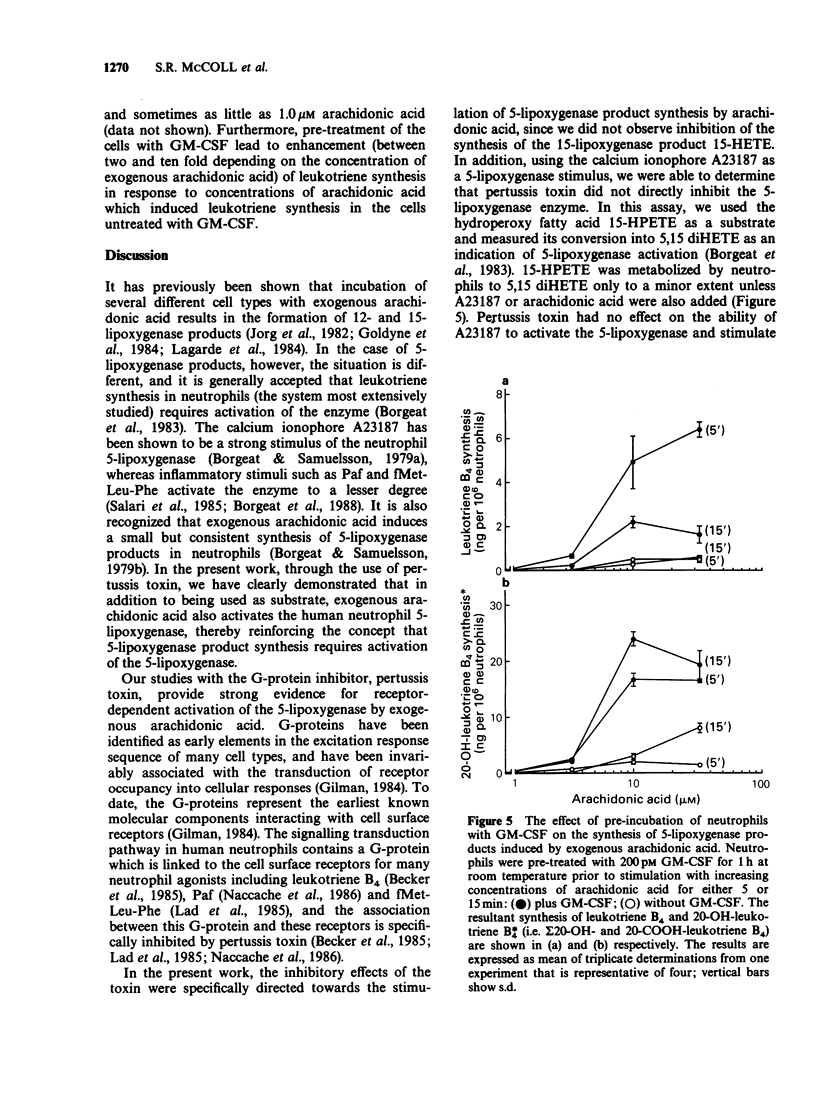
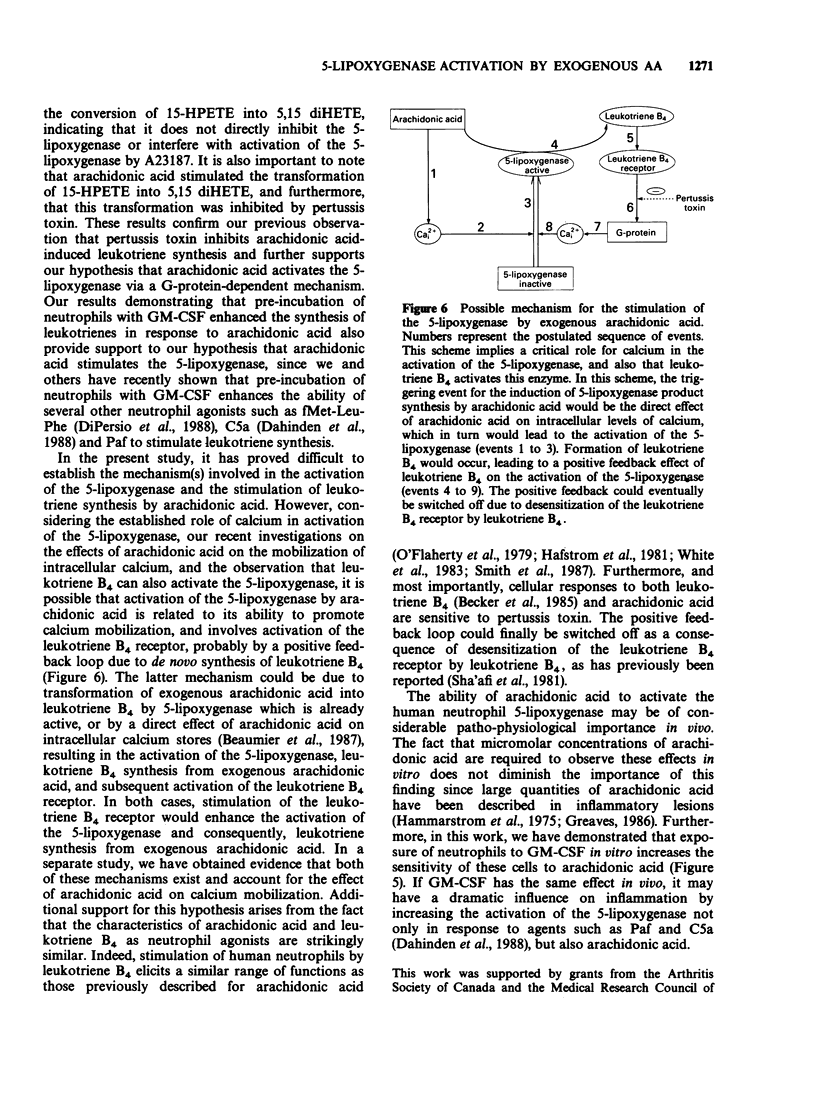
1. The mechanism by which incubation of human peripheral blood neutrophils with exogenous arachidonic acid leads to 5-lipoxygenase product synthesis was investigated. 2. Incubation of neutrophils with arachidonic acid caused a concentration- and time-dependent synthesis of leukotriene B4, its omega-oxidation products, and 5-hydroxyeicosatetraenoic acid. 3. The threshold concentration of arachidonic acid required for this effect was equal to, or greater than 3.3 microM and the synthesis increased with up to 33 microM arachidonic acid, the highest concentration used. Synthesis induced by arachidonic acid increased with time for up to 15 min and the major products detected were the omega-oxidation products of leukotriene B4. 4. Pre-incubation of neutrophils with pertussis toxin inhibited the synthesis of 5-lipoxygenase products induced by arachidonic acid by 75% or more, but had no effect on either arachidonic acid-induced synthesis of the 15-lipoxygenase product, 15-hydroxyeicosatetraenoic acid, or activation of the 5-lipoxygenase induced by the calcium ionophore A23187. 5. Pre-incubation of neutrophils with granulocyte-macrophage colony-stimulating factor lead to enhanced leukotriene synthesis in response to arachidonic acid. 6. These results imply that exogenous arachidonic acid is not only used as a substrate, but also activates the 5-lipoxygenase. Possible mechanisms of action are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badwey J. A., Curnutte J. T., Robinson J. M., Berde C. B., Karnovsky M. J., Karnovsky M. L. Effects of free fatty acids on release of superoxide and on change of shape by human neutrophils. Reversibility by albumin. J Biol Chem. 1984 Jun 25;259(12):7870–7877. [PubMed] [Google Scholar]
- Beaumier L., Faucher N., Naccache P. H. Arachidonic acid-induced release of calcium in permeabilized human neutrophils. FEBS Lett. 1987 Sep 14;221(2):289–292. doi: 10.1016/0014-5793(87)80942-0. [DOI] [PubMed] [Google Scholar]
- Becker E. L., Kermode J. C., Naccache P. H., Yassin R., Marsh M. L., Munoz J. J., Sha'afi R. I. The inhibition of neutrophil granule enzyme secretion and chemotaxis by pertussis toxin. J Cell Biol. 1985 May;100(5):1641–1646. doi: 10.1083/jcb.100.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Fruteau de Laclos B., Maclouf J. New concepts in the modulation of leukotriene synthesis. Biochem Pharmacol. 1983 Feb 1;32(3):381–387. doi: 10.1016/0006-2952(83)90515-4. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Picard S. 19-Hydroxyprostaglandin B2 as an internal standard for on-line extraction-high-performance liquid chromatography analysis of lipoxygenase products. Anal Biochem. 1988 Jun;171(2):283–289. doi: 10.1016/0003-2697(88)90487-3. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: effects of ionophore A23187. Proc Natl Acad Sci U S A. 1979 May;76(5):2148–2152. doi: 10.1073/pnas.76.5.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Metabolism of arachidonic acid in polymorphonuclear leukocytes. Structural analysis of novel hydroxylated compounds. J Biol Chem. 1979 Aug 25;254(16):7865–7869. [PubMed] [Google Scholar]
- Bradford P. G., Rubin R. P. Pertussis toxin inhibits chemotactic factor-induced phospholipase C stimulation and lysosomal enzyme secretion in rabbit neutrophils. FEBS Lett. 1985 Apr 22;183(2):317–320. doi: 10.1016/0014-5793(85)80801-2. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Clark R. A., Leidal K. G., Pearson D. W., Nauseef W. M. NADPH oxidase of human neutrophils. Subcellular localization and characterization of an arachidonate-activatable superoxide-generating system. J Biol Chem. 1987 Mar 25;262(9):4065–4074. [PubMed] [Google Scholar]
- Cox J. A., Jeng A. Y., Blumberg P. M., Tauber A. I. Comparison of subcellular activation of the human neutrophil NADPH-oxidase by arachidonic acid, sodium dodecyl sulfate (SDS), and phorbol myristate acetate (PMA). J Immunol. 1987 Mar 15;138(6):1884–1888. [PubMed] [Google Scholar]
- Dahinden C. A., Zingg J., Maly F. E., de Weck A. L. Leukotriene production in human neutrophils primed by recombinant human granulocyte/macrophage colony-stimulating factor and stimulated with the complement component C5A and FMLP as second signals. J Exp Med. 1988 Apr 1;167(4):1281–1295. doi: 10.1084/jem.167.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio J. F., Naccache P. H., Borgeat P., Gasson J. C., Nguyen M. H., McColl S. R. Characterization of the priming effects of human granulocyte-macrophage colony-stimulating factor on human neutrophil leukotriene synthesis. Prostaglandins. 1988 Nov;36(5):673–691. doi: 10.1016/0090-6980(88)90013-5. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Goldman D. W., Goetzl E. J. Heterogeneity of human polymorphonuclear leukocyte receptors for leukotriene B4. Identification of a subset of high affinity receptors that transduce the chemotactic response. J Exp Med. 1984 Apr 1;159(4):1027–1041. doi: 10.1084/jem.159.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldyne M. E., Burrish G. F., Poubelle P., Borgeat P. Arachidonic acid metabolism among human mononuclear leukocytes. Lipoxygenase-related pathways. J Biol Chem. 1984 Jul 25;259(14):8815–8819. [PubMed] [Google Scholar]
- Hafstrom I., Palmblad J., Malmsten C. L., Rådmark O., Samuelsson B. Leukotriene B4--a stereospecific stimulator for release of lysosomal enzymes from neutrophils. FEBS Lett. 1981 Jul 20;130(1):146–148. doi: 10.1016/0014-5793(81)80684-9. [DOI] [PubMed] [Google Scholar]
- Hammarström S., Hamberg M., Samuelsson B., Duell E. A., Stawiski M., Voorhees J. J. Increased concentrations of nonesterified arachidonic acid, 12L-hydroxy-5,8,10,14-eicosatetraenoic acid, prostaglandin E2, and prostaglandin F2alpha in epidermis of psoriasis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5130–5134. doi: 10.1073/pnas.72.12.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörg A., Henderson W. R., Murphy R. C., Klebanoff S. J. Leukotriene generation by eosinophils. J Exp Med. 1982 Feb 1;155(2):390–402. doi: 10.1084/jem.155.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad P. M., Olson C. V., Grewal I. S., Scott S. J. A pertussis toxin-sensitive GTP-binding protein in the human neutrophil regulates multiple receptors, calcium mobilization, and lectin-induced capping. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8643–8647. doi: 10.1073/pnas.82.24.8643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde M., Croset M., Boukhchache D., Greffe A., Dechavanne M., Renaud S. Lipoxygenase activity of intact human platelets. Prostaglandins Leukot Med. 1984 Jan;13(1):61–66. doi: 10.1016/0262-1746(84)90103-3. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. The biologically active leukotrienes. Biosynthesis, metabolism, receptors, functions, and pharmacology. J Clin Invest. 1984 Apr;73(4):889–897. doi: 10.1172/JCI111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmsten C. L., Palmblad J., Udén A. M., Rådmark O., Engstedt L., Samuelsson B. Leukotriene B4: a highly potent and stereospecific factor stimulating migration of polymorphonuclear leukocytes. Acta Physiol Scand. 1980 Dec;110(4):449–451. doi: 10.1111/j.1748-1716.1980.tb06696.x. [DOI] [PubMed] [Google Scholar]
- Maridonneau-Parini I., Tauber A. I. Activation of NADPH-oxidase by arachidonic acid involves phospholipase A2 in intact human neutrophils but not in the cell-free system. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1099–1105. doi: 10.1016/s0006-291x(86)80395-3. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984 May 11;224(4649):622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Molski M. M., Volpi M., Shefcyk J., Molski T. F., Loew L., Becker E. L., Sha'afi R. I. Biochemical events associated with the stimulation of rabbit neutrophils by platelet-activating factor. J Leukoc Biol. 1986 Nov;40(5):533–548. doi: 10.1002/jlb.40.5.533. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Sha'afi R. I., Borgeat P., Goetzl E. J. Mono- and dihydroxyeicosatetraenoic acids alter calcium homeostasis in rabbit neutrophils. J Clin Invest. 1981 May;67(5):1584–1587. doi: 10.1172/JCI110191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Becker E. L., Ward P. A. Neutrophil aggregation and degranulation. Effect of arachidonic acid. Am J Pathol. 1979 May;95(2):433–444. [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A., Kargman S. Translocation of 5-lipoxygenase to the membrane in human leukocytes challenged with ionophore A23187. J Biol Chem. 1988 Aug 5;263(22):10980–10988. [PubMed] [Google Scholar]
- Rouzer C. A., Samuelsson B. Reversible, calcium-dependent membrane association of human leukocyte 5-lipoxygenase. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7393–7397. doi: 10.1073/pnas.84.21.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salari H., Braquet P., Naccache P., Borgeat P. Characterization of effect of N-formyl-methionyl-leucyl-phenylalanine on leukotriene synthesis in human polymorphonuclear leukocytes. Inflammation. 1985 Jun;9(2):127–138. doi: 10.1007/BF00917585. [DOI] [PubMed] [Google Scholar]
- Samuelsson B., Dahlén S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987 Sep 4;237(4819):1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Sha'afi R. I., Molski T. F., Borgeat P., Naccache P. H. Deactivation of the effects of F-Met-Leu-Phe and leukotriene B4 on calcium mobilization in rabbit neutrophils. Biochem Biophys Res Commun. 1981 Nov 30;103(2):766–773. doi: 10.1016/0006-291x(81)90515-5. [DOI] [PubMed] [Google Scholar]
- Sha'afi R. I., Naccache P. H., Alobaidi T., Molski T. F., Volpi M. Effect of arachidonic acid and the chemotactic factor F-Met-Leu-Phe on cation transport in rabbit neutrophils. J Cell Physiol. 1981 Feb;106(2):215–223. doi: 10.1002/jcp.1041060207. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Sam L. M., Justen J. M., Leach K. L., Epps D. E. Human polymorphonuclear neutrophil activation with arachidonic acid. Br J Pharmacol. 1987 Jul;91(3):641–649. doi: 10.1111/j.1476-5381.1987.tb11258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. R., Naccache P. H., Molski T. F., Borgeat P., Sha'afi R. I. Direct demonstration of increased intracellular concentration of free calcium in rabbit and human neutrophils following stimulation by chemotactic factor. Biochem Biophys Res Commun. 1983 May 31;113(1):44–50. doi: 10.1016/0006-291x(83)90429-1. [DOI] [PubMed] [Google Scholar]


