Abstract
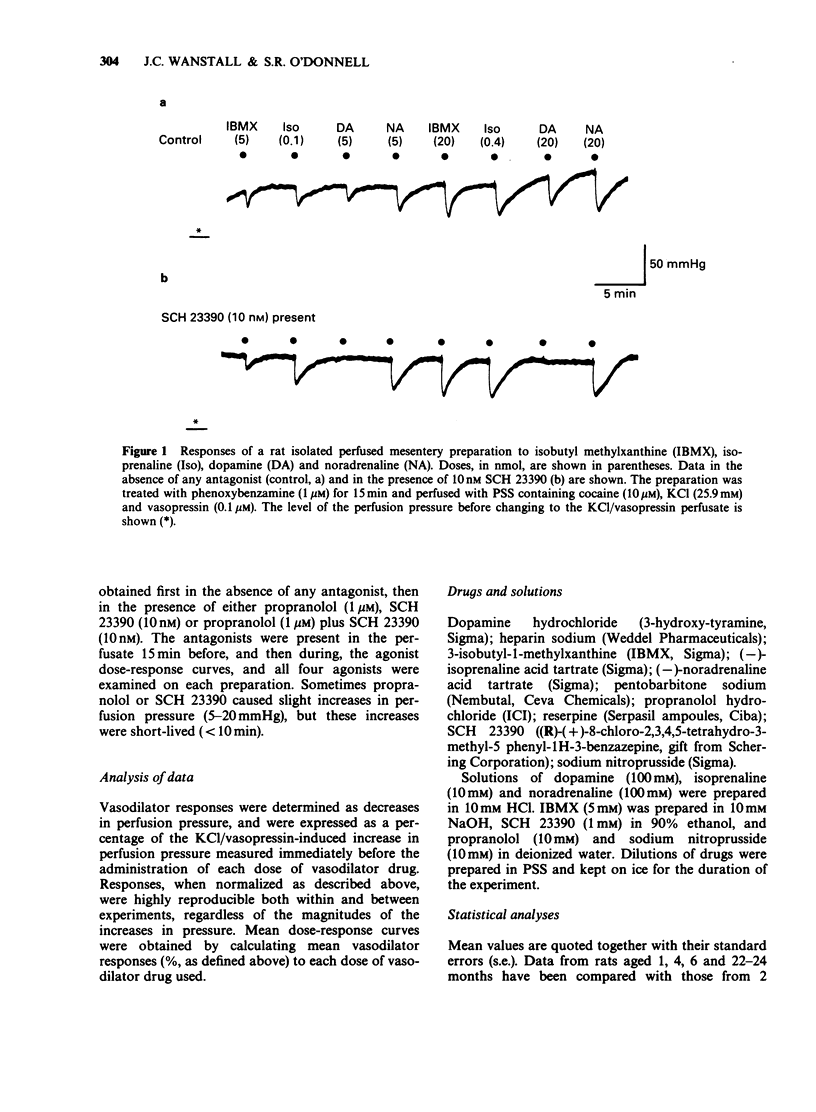
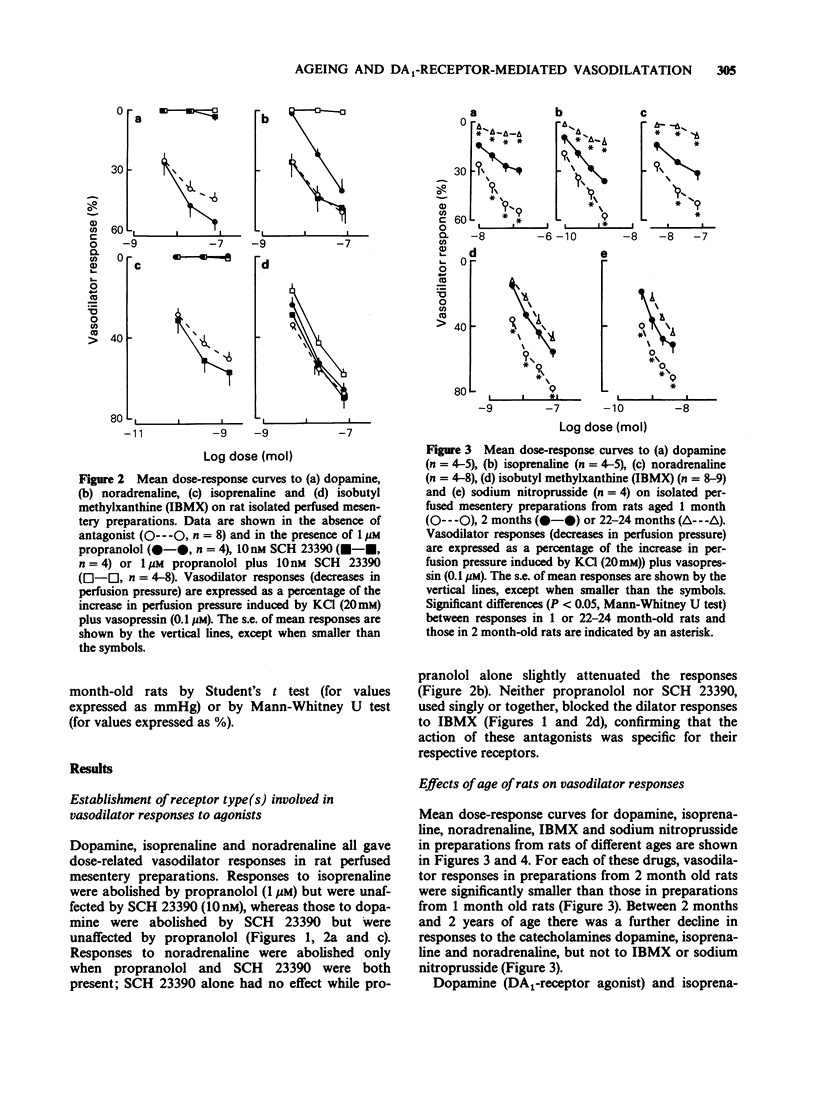
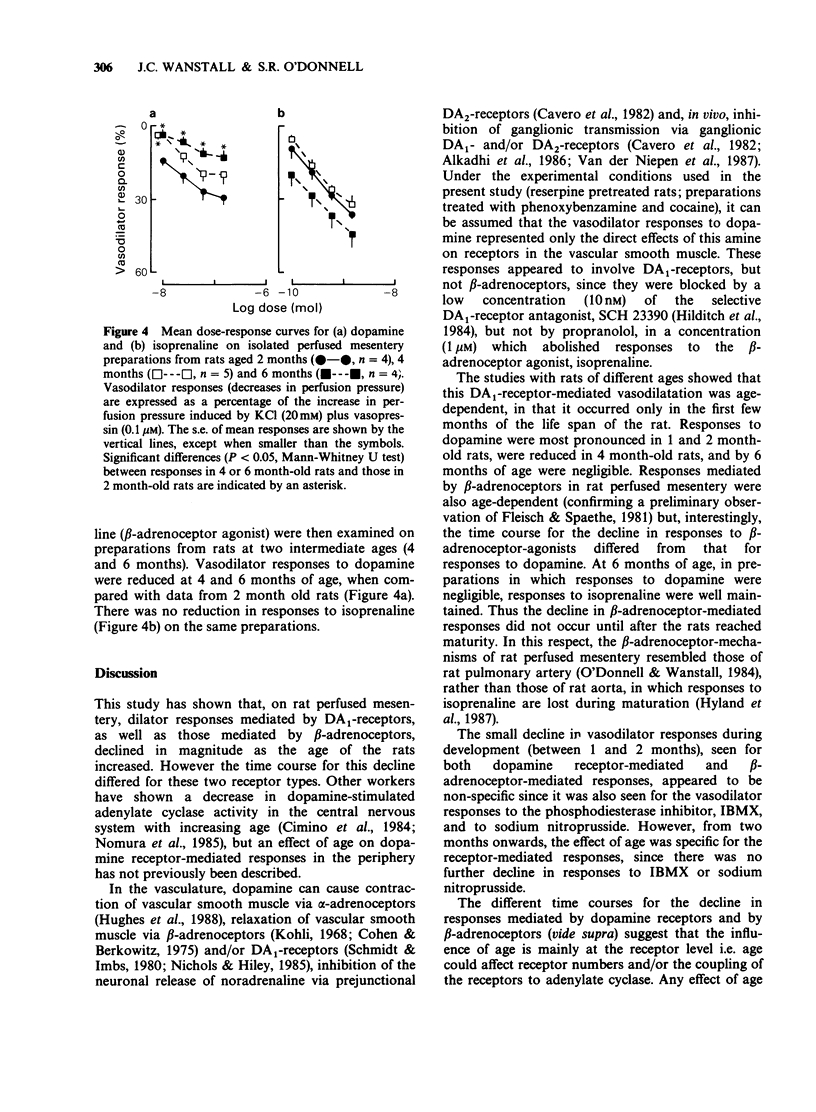
1. Dose-dependent vasodilator responses to dopamine, isoprenaline, noradrenaline, 3-isobutyl-1-methylxanthine (IBMX) and sodium nitroprusside were obtained in isolated perfused mesentery preparations, taken from reserpine-treated rats of different ages. The preparations were pretreated with phenoxybenzamine (1 microM) and perfused with physiological salt solution containing cocaine (10 microM), additional KCl (20 mM) and vasopressin (0.1 microM). 2. Vasodilator responses to dopamine were abolished by the dopamine1 (DA1)-selective antagonist SCH 23390 (10 nM) and those to isoprenaline by propranolol (1 microM), but the vasodilator responses to noradrenaline were abolished only when SCH 23390 and propranolol were used together. This indicated that dopamine was acting via DA1-receptors, isoprenaline via beta-adrenoceptors and that noradrenaline could act via DA1-receptors and beta-adrenoceptors in this preparation. 3. Responses to all the vasodilator drugs decreased in magnitude between the ages of 1 and 2 months. Responses to dopamine declined further in 4 month-old rats and were negligible at 6 or 22-24 months of age. Responses to isoprenaline were well maintained up to 6 months of age, but were negligible at 22-24 months. 4. It is concluded that, in the rat mesenteric vasculature, there is a non-specific decline in responses to vasodilator drugs during development (1 to 2 months). Subsequently there is a specific decline in DA1-receptor-mediated and beta-adrenoceptor-mediated responses; the former are lost at an earlier age than the latter. This different time course suggests that age influences receptor numbers, or their coupling to adenylate cyclase, rather than a post-receptor event in the adenylate cyclase/cyclic AMP pathway.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkadhi K. A., Sabouni M. H., Ansari A. F., Lokhandwala M. F. Activation of DA1 receptors by dopamine or fenoldopam increases cyclic AMP levels in the renal artery but not in the superior cervical ganglion of the rat. J Pharmacol Exp Ther. 1986 Aug;238(2):547–553. [PubMed] [Google Scholar]
- Amenta F., Cavallotti C., De Rossi M., Mione M. C. Dopamine-sensitive cAMP generating system in rat extracerebral arteries. Eur J Pharmacol. 1984 Jan 13;97(1-2):105–109. doi: 10.1016/0014-2999(84)90517-x. [DOI] [PubMed] [Google Scholar]
- Caruana M. P., Heber M., Brigden G., Raftery E. B. Effects of fenoldopam, a specific dopamine receptor agonist, on blood pressure and left ventricular function in systemic hypertension. Br J Clin Pharmacol. 1987 Dec;24(6):721–727. doi: 10.1111/j.1365-2125.1987.tb03237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavero I., Massingham R., Lefèvre-Borg F. Peripheral dopamine receptors, potential targets for a new class of antihypertensive agents. Part I: Subclassification and functional description. Life Sci. 1982 Sep 6;31(10):939–948. doi: 10.1016/0024-3205(82)90165-5. [DOI] [PubMed] [Google Scholar]
- Cimino M., Vantini G., Algeri S., Curatola G., Pezzoli C., Stramentinoli G. Age-related modification of dopaminergic and beta-Adrenergic receptor system: restoration to normal activity by modifying membrane fluidity with S-adenosylmethionine. Life Sci. 1984 May 21;34(21):2029–2039. doi: 10.1016/0024-3205(84)90367-9. [DOI] [PubMed] [Google Scholar]
- Cohen M. L., Berkowitz B. A. Differences between the effects of dopamine and apomorphine on rat aortic strips. Eur J Pharmacol. 1975 Nov;34(1):49–58. doi: 10.1016/0014-2999(75)90224-1. [DOI] [PubMed] [Google Scholar]
- Crooks R. J., Martin G. R. An isolated vascular tissue preparation showing a specific relaxant effect of dopamine [proceedings]. Br J Pharmacol. 1979 Nov;67(3):474P–474P. [PMC free article] [PubMed] [Google Scholar]
- Dupont A. G., Lefebvre R. A., Vanderniepen P. Influence of the dopamine receptor agonists fenoldopam and quinpirole in the rat superior mesenteric vascular bed. Br J Pharmacol. 1987 Jul;91(3):493–501. doi: 10.1111/j.1476-5381.1987.tb11242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch J. H., Spaethe S. M. Vasodilation and aging evaluated in the isolated perfused rat mesenteric vascular bed: preliminary observations on the vascular pharmacology of dobutamine. J Cardiovasc Pharmacol. 1981 Jan-Feb;3(1):187–196. doi: 10.1097/00005344-198101000-00017. [DOI] [PubMed] [Google Scholar]
- Goldberg L. I., Volkman P. H., Kohli J. D. A comparison of the vascular dopamine receptor with other dopamine receptors. Annu Rev Pharmacol Toxicol. 1978;18:57–79. doi: 10.1146/annurev.pa.18.040178.000421. [DOI] [PubMed] [Google Scholar]
- Harvey J. N., Worth D. P., Brown J., Lee M. R. The effect of oral fenoldopam (SKF 82526-J), a peripheral dopamine receptor agonist, on blood pressure and renal function in normal man. Br J Clin Pharmacol. 1985 Jan;19(1):21–27. doi: 10.1111/j.1365-2125.1985.tb02608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilditch A., Drew G. M., Naylor R. J. SCH 23390 is a very potent and selective antagonist at vascular dopamine receptors. Eur J Pharmacol. 1984 Jan 27;97(3-4):333–334. doi: 10.1016/0014-2999(84)90471-0. [DOI] [PubMed] [Google Scholar]
- Hughes A., Inkpen P., Sever P. Action of dopamine on isolated human saphenous veins. J Cardiovasc Pharmacol. 1988 Mar;11(3):373–377. doi: 10.1097/00005344-198803000-00016. [DOI] [PubMed] [Google Scholar]
- Hughes A., Thom S., Martin G., Redman D., Hasan S., Sever P. The action of a dopamine (DA1) receptor agonist, fenoldopam in human vasculature in vivo and in vitro. Br J Clin Pharmacol. 1986 Nov;22(5):535–540. doi: 10.1111/j.1365-2125.1986.tb02932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland L., Warnock P., Docherty J. R. Age-related alterations in alpha 1- and beta-adrenoceptors mediated responsiveness of rat aorta. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jan;335(1):50–53. doi: 10.1007/BF00165035. [DOI] [PubMed] [Google Scholar]
- Kohli J. D. A comparative study of dopamine and noradrenaline on the rabbit aorta. Can J Physiol Pharmacol. 1969 Feb;47(2):171–174. doi: 10.1139/y69-029. [DOI] [PubMed] [Google Scholar]
- Mousdale S., Clyburn P. A., Mackie A. M., Groves N. D., Rosen M. Comparison of the effects of dopamine, dobutamine, and dopexamine upon renal blood flow: a study in normal healthy volunteers. Br J Clin Pharmacol. 1988 May;25(5):555–560. doi: 10.1111/j.1365-2125.1988.tb03345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols A. J., Hiley C. R. Identification of adrenoceptors and dopamine receptors mediating vascular responses in the superior mesenteric arterial bed of the rat. J Pharm Pharmacol. 1985 Feb;37(2):110–115. doi: 10.1111/j.2042-7158.1985.tb05017.x. [DOI] [PubMed] [Google Scholar]
- Nomura Y., Makihata J., Segawa T. Activation of adenylate cyclase by dopamine, GTP, NaF and forskolin in striatal membranes of neonatal, adult and senescent rats. Eur J Pharmacol. 1984 Nov 13;106(2):437–440. doi: 10.1016/0014-2999(84)90736-2. [DOI] [PubMed] [Google Scholar]
- O'Donnell S. R., Wanstall J. C. Beta-1 and beta-2 adrenoceptor-mediated responses in preparations of pulmonary artery and aorta from young and aged rats. J Pharmacol Exp Ther. 1984 Mar;228(3):733–738. [PubMed] [Google Scholar]
- O'Donnell S. R., Wanstall J. C. Choice and concentration of contractile agent influence responses of rat aorta to vascular relaxant drugs. J Pharm Pharmacol. 1987 Oct;39(10):848–850. doi: 10.1111/j.2042-7158.1987.tb05132.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell S. R., Wanstall J. C. Thyroxine treatment of aged or young rats demonstrates that vascular responses mediated by beta-adrenoceptor subtypes can be differentially regulated. Br J Pharmacol. 1986 May;88(1):41–49. doi: 10.1111/j.1476-5381.1986.tb09469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil P. N., Miller D. D., Trendelenburg U. Molecular geometry and adrenergic drug activity. Pharmacol Rev. 1974 Dec;26(4):323–392. [PubMed] [Google Scholar]
- Schmidt M., Imbs J. L. Pharmacological characterization of renal vascular dopamine receptors. J Cardiovasc Pharmacol. 1980 Sep-Oct;2(5):595–605. doi: 10.1097/00005344-198009000-00009. [DOI] [PubMed] [Google Scholar]
- Toda N., Goldberg L. I. Effects of dopamine on isolated canine coronary arteries. Cardiovasc Res. 1975 May;9(3):384–389. doi: 10.1093/cvr/9.3.384. [DOI] [PubMed] [Google Scholar]
- Toda N. Influence of dopamine and noradrenaline on isolated cerebral arteries of the dog. Br J Pharmacol. 1976 Sep;58(1):121–126. doi: 10.1111/j.1476-5381.1976.tb07700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto G., Lee C. H., Hoffman B. B. Age-related decrease in beta adrenergic receptor-mediated vascular smooth muscle relaxation. J Pharmacol Exp Ther. 1986 Nov;239(2):411–415. [PubMed] [Google Scholar]
- Van der Niepen P., Lefebvre R. A., Dupont A. G. Neurogenic vasodilatation produced by fenoldopam in the rat hindquarters vascular bed. J Auton Pharmacol. 1987 Dec;7(4):331–338. doi: 10.1111/j.1474-8673.1987.tb00161.x. [DOI] [PubMed] [Google Scholar]


