Abstract
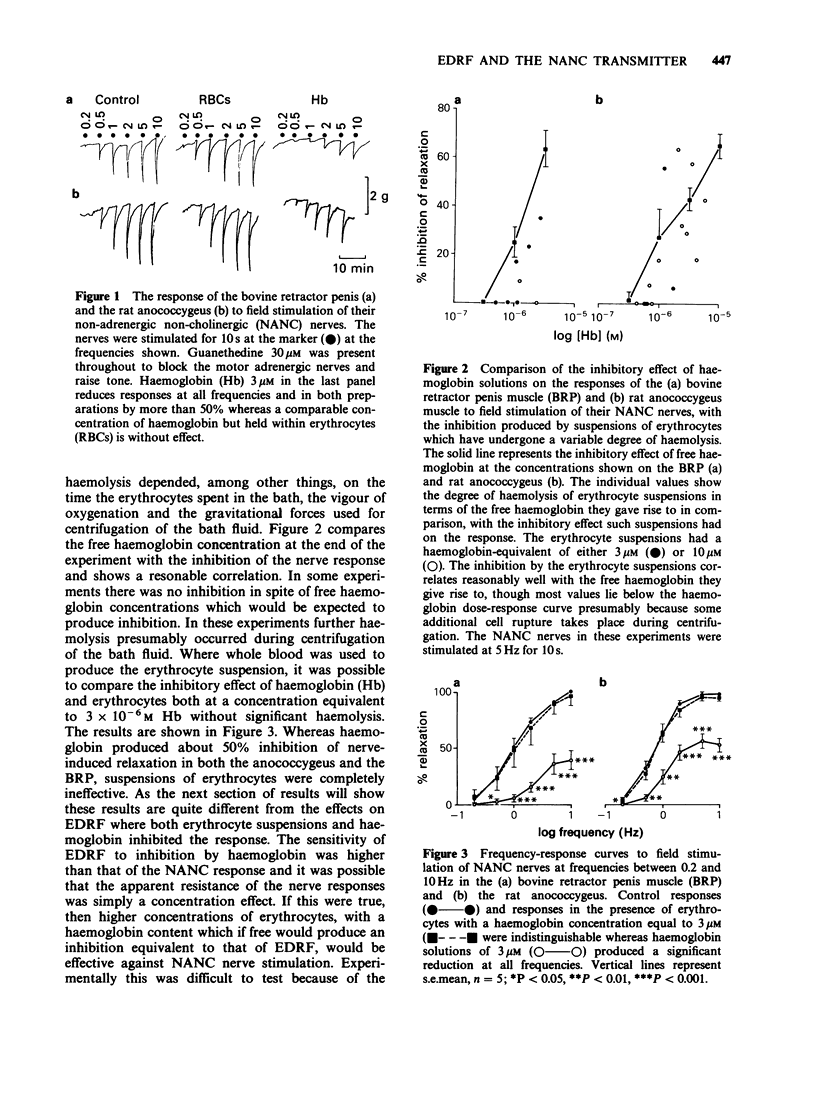
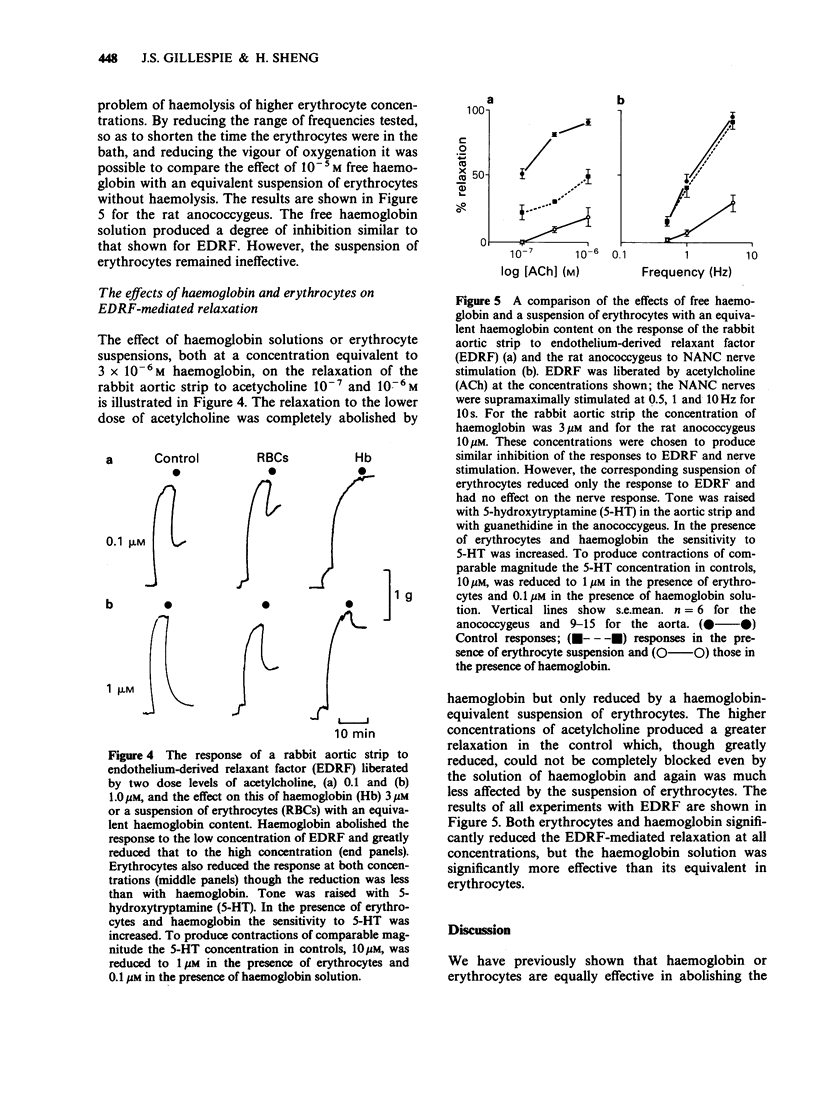
1. The inhibitory effect of erythrocyte suspensions and haemoglobin solutions on the response of the bovine retractor penis muscle (BRP) and the rat anococcygeus to field stimulation of their non-adrenergic non-cholinergic (NANC) nerves has been compared. Haemoglobin 3 microM greatly reduced the relaxant response in both tissues whereas a haemoglobin-equivalent suspension of erythrocytes was without effect. 2. A similar comparison of erythrocytes and haemoglobin on the response of the rabbit aortic strip to EDRF liberated by acetylcholine (ACh) showed that both reduced EDRF-mediated relaxation, though haemoglobin was significantly more effective. 3. These results suggest that the NANC transmitter may not be as freely diffusible through the erythrocyte membrane as EDRF and may therefore not be nitric oxide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman A., Drummond A. H. Cyclic GMP mediates neurogenic relaxation in the bovine retractor penis muscle. Br J Pharmacol. 1984 Apr;81(4):665–674. doi: 10.1111/j.1476-5381.1984.tb16133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S. Block of some non-adrenergic inhibitory responses of smooth muscle by a substance from haemolysed erythrocytes. J Physiol. 1982 Jul;328:11–25. doi: 10.1113/jphysiol.1982.sp014250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Pollock D. Oxyhaemoglobin blocks non-adrenergic non-cholinergic inhibition in the bovine retractor penis muscle. Eur J Pharmacol. 1982 Nov 19;85(2):221–224. doi: 10.1016/0014-2999(82)90470-8. [DOI] [PubMed] [Google Scholar]
- Bowman A., McGrath J. C. The effect of hypoxia on neuroeffector transmission in the bovine retractor penis and rat anococcygeus muscles. Br J Pharmacol. 1985 Aug;85(4):869–875. doi: 10.1111/j.1476-5381.1985.tb11086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J., Chu E. B. Possible role for cyclic GMP in endothelium-dependent relaxation of rabbit aorta by acetylcholine. Comparison with nitroglycerin. Res Commun Chem Pathol Pharmacol. 1983 Sep;41(3):369–381. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Lüllmann-Rauch R. On the ultrastructure of the rat anococcygeus muscle. Cell Tissue Res. 1974;149(1):91–104. doi: 10.1007/BF00209052. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Sheng H. Influence of haemoglobin and erythrocytes on the effects of EDRF, a smooth muscle inhibitory factor, and nitric oxide on vascular and non-vascular smooth muscle. Br J Pharmacol. 1988 Dec;95(4):1151–1156. doi: 10.1111/j.1476-5381.1988.tb11750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S. The rat anococcygeus muscle and its response to nerve stimulation and to some drugs. Br J Pharmacol. 1972 Jul;45(3):404–416. doi: 10.1111/j.1476-5381.1972.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]


