Abstract
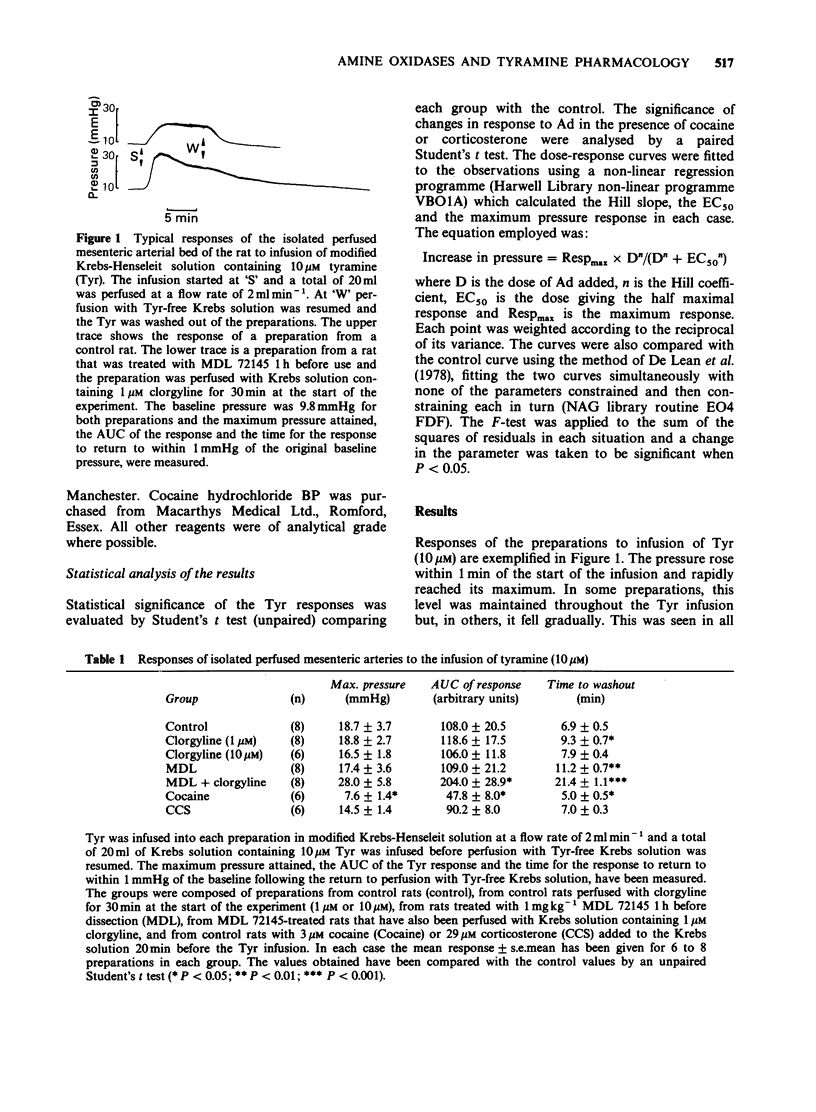
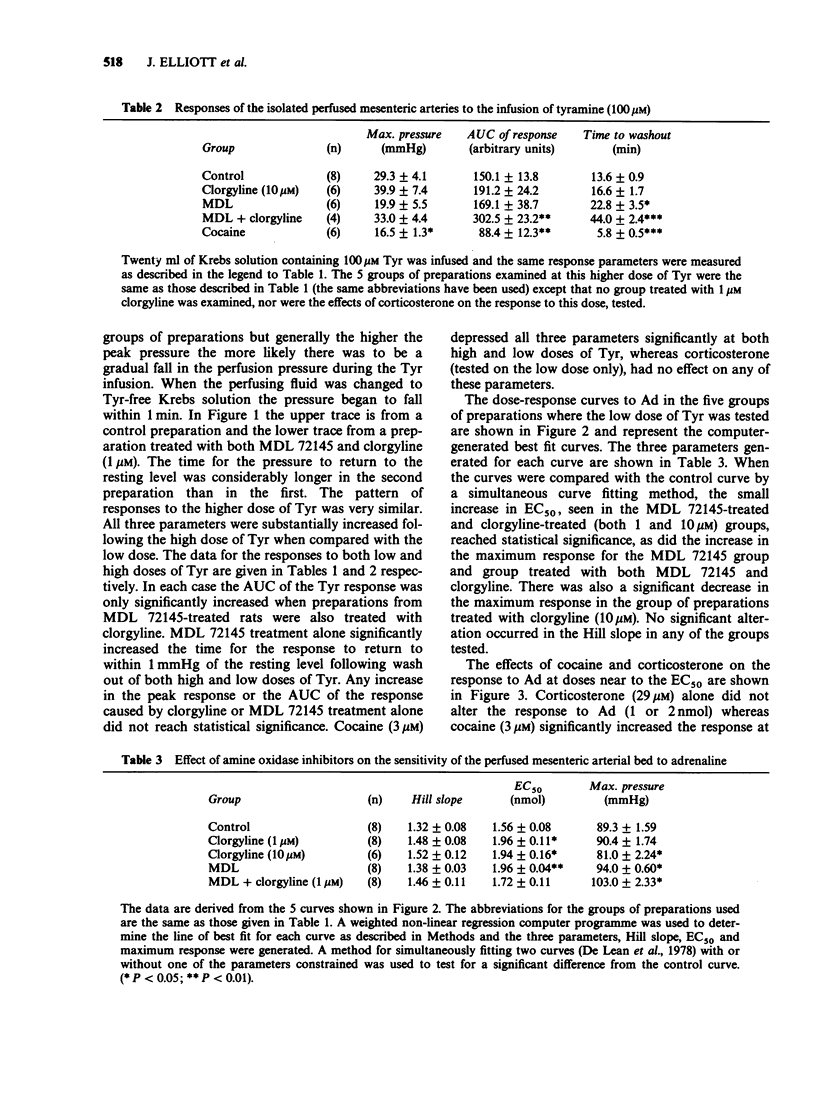
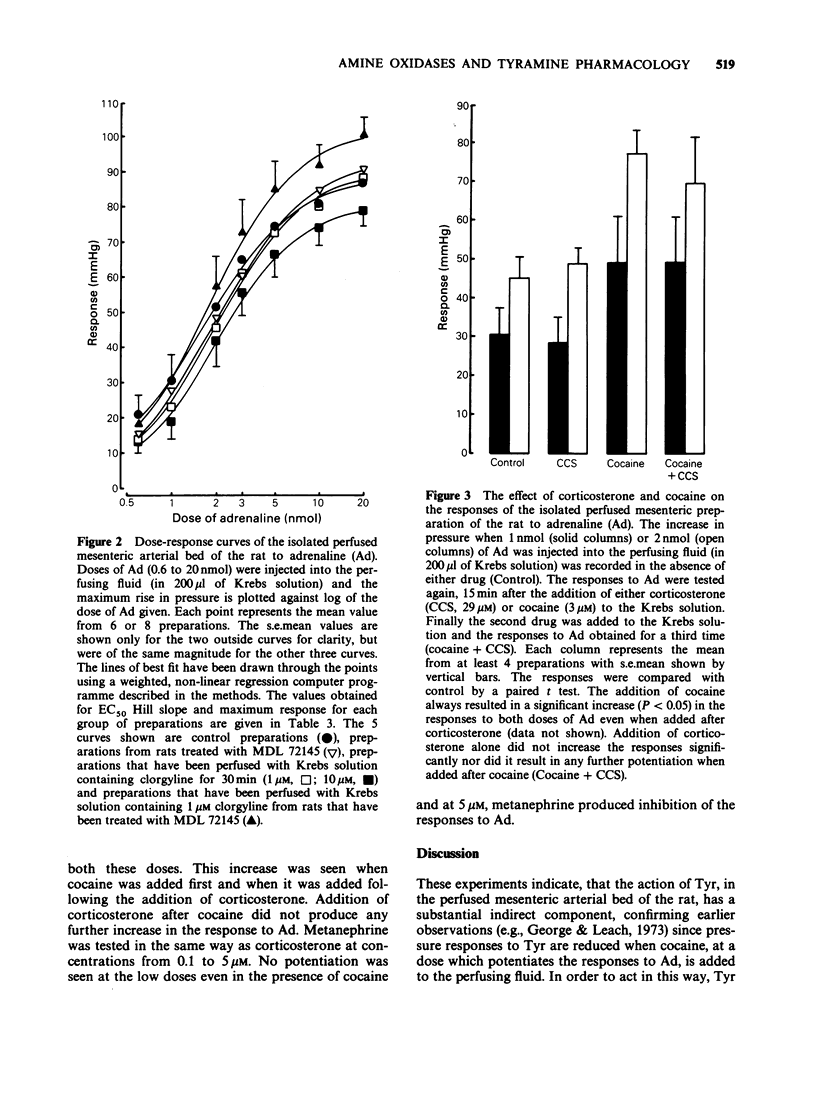
1. The pressor response to the infusion of tyramine (Tyr) into the isolated perfused mesenteric arterial bed of the rat has been studied at both a low and a high dose (0.2 and 2.0 mumol) and the effect of monoamine oxidase-A (MAO-A) and semicarbazide-sensitive amine oxidase (SSAO) inhibition was examined. Very little MAO-B activity is found in homogenates of this tissue when Tyr is used as substrate. 2. Inhibition of SSAO by treating rats with 1 mg kg-1 (E)-2-(3',4'-dimethoxyphenyl)-3-fluoroally lamine (MDL 72145) 1 h before dissection, had no significant effect on the maximum pressure attained or the area under the curve (AUC) of the response to both low and high doses of Tyr. Inhibition of MAO-A, by inclusion of 10 microM clorgyline in the perfusing fluid, resulted in no significant potentiation at both low or high doses of Tyr. The inhibition of both these enzymes together substantially increased the AUC of the pressor response. 3. Cocaine (3 microM) significantly potentiated the responses to adrenaline (Ad). At this dose, cocaine significantly reduced the peak height and the AUC of the responses to both doses of Tyr. 4. Inhibition of extraneuronal uptake mechanisms with corticosterone (29 microM) did not potentiate the response to Ad and did not significantly alter the response to Tyr (low dose). 5. The effects of MDL 72145 and clorgyline on the directly acting amine, Ad, were studied. MDL 72145 caused a small but significant increase in the EC50 and in the maximum response to Ad, whilst clorgyline (10 microM) increased the EC50 value slightly and decreased the maximum response.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACKWELL B. HYPERTENSIVE CRISIS DUE TO MONOAMINE-OXIDASE INHIBITORS. Lancet. 1963 Oct 26;2(7313):849–850. doi: 10.1016/s0140-6736(63)92743-0. [DOI] [PubMed] [Google Scholar]
- Coquil J. F., Goridis C., Mack G., Neff N. H. Monoamine oxidase in rat arteries: evidence for different forms and selective localization. Br J Pharmacol. 1973 Aug;48(4):590–599. doi: 10.1111/j.1476-5381.1973.tb08245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Lande I. S., Hill B. D., Jellett L. B., McNeil J. M. The role of monoamine oxidase in the response of the isolated central artery of the rabbit ear to tyramine. Br J Pharmacol. 1970 Oct;40(2):249–256. doi: 10.1111/j.1476-5381.1970.tb09918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Elliott J., Callingham B. A., Sharman D. F. Metabolism of amines in the isolated perfused mesenteric arterial bed of the rat. Br J Pharmacol. 1989 Oct;98(2):507–514. doi: 10.1111/j.1476-5381.1989.tb12624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finberg J. P., Tenne M. Relationship between tyramine potentiation and selective inhibition of monoamine oxidase types A and B in the rat vas deferens. Br J Pharmacol. 1982 Sep;77(1):13–21. doi: 10.1111/j.1476-5381.1982.tb09263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozard J. R., Zreika M., Robin M., Palfreyman M. G. The functional consequences of inhibition of monoamine oxidase type B: comparison of the pharmacological properties of L-deprenyl and MDL 72145. Naunyn Schmiedebergs Arch Pharmacol. 1985 Nov;331(2-3):186–193. doi: 10.1007/BF00634237. [DOI] [PubMed] [Google Scholar]
- George A. J., Leach G. D. The effects of changes in ionic environment and modification of adrenergic function on the vascular responses to sympathomimetic amines. J Pharm Pharmacol. 1973 Jul;25(7):521–529. doi: 10.1111/j.2042-7158.1973.tb09151.x. [DOI] [PubMed] [Google Scholar]
- George A. J., Leach G. D. The involvement of Ca2+ and Mg2+ in the spontaneous and drug induced release of (3H)noradrenaline from mesenteric arteries. Biochem Pharmacol. 1975 Mar 15;24(6):737–741. doi: 10.1016/0006-2952(75)90252-x. [DOI] [PubMed] [Google Scholar]
- Greenawalt J. W., Schnaitman C. An appraisal of the use of monoamine oxidase as an enzyme marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1970 Jul;46(1):173–179. doi: 10.1083/jcb.46.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge R. L., Robinson S. M. The action of clonidine on isolated arterial preparations. Aust J Exp Biol Med Sci. 1972 Aug;50(4):517–526. doi: 10.1038/icb.1972.44. [DOI] [PubMed] [Google Scholar]
- Iriarte C. F., Pascual R., Morcillo E., Bevan J. A. Responses of rabbit aorta to tyramine in relation to the surface of drug entry. Naunyn Schmiedebergs Arch Pharmacol. 1985 Mar;329(1):24–29. doi: 10.1007/BF00695187. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Salt P. J. Inhibition of catecholamine Uptake-2 by steroids in the isolated rat heart. Br J Pharmacol. 1970 Nov;40(3):528–530. doi: 10.1111/j.1476-5381.1970.tb10637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrott B., Iversen L. L. Noradrenaline metabolizing enzymes in normal and sympathetically denervated vas deferens. J Neurochem. 1971 Jan;18(1):1–6. doi: 10.1111/j.1471-4159.1971.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Krishnamurty V. S., Grollman A. Contractile response of rat aorta to norepinephrine and tyramine. J Pharmacol Exp Ther. 1972 Aug;182(2):264–272. [PubMed] [Google Scholar]
- Lewinsohn R. Amine oxidase in human blood vessels and non-vascular smooth muscle. J Pharm Pharmacol. 1981 Sep;33(9):569–575. doi: 10.1111/j.2042-7158.1981.tb13868.x. [DOI] [PubMed] [Google Scholar]
- Lyles G. A., Singh I. Vascular smooth muscle cells: a major source of the semicarbazide-sensitive amine oxidase of the rat aorta. J Pharm Pharmacol. 1985 Sep;37(9):637–643. doi: 10.1111/j.2042-7158.1985.tb05100.x. [DOI] [PubMed] [Google Scholar]
- Miyahara H., Suzuki H. Effects of tyramine on noradrenaline outflow and electrical responses induced by field stimulation in the perfused rabbit ear artery. Br J Pharmacol. 1985 Oct;86(2):405–416. doi: 10.1111/j.1476-5381.1985.tb08910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precious E., Lyles G. A. Properties of a semicarbazide-sensitive amine oxidase in human umbilical artery. J Pharm Pharmacol. 1988 Sep;40(9):627–633. doi: 10.1111/j.2042-7158.1988.tb05322.x. [DOI] [PubMed] [Google Scholar]
- Rucker R. B., Goettlich-Riemann W. Properties of rabbit aorta amine oxidase. Proc Soc Exp Biol Med. 1972 Jan;139(1):286–289. doi: 10.3181/00379727-139-36127. [DOI] [PubMed] [Google Scholar]
- Toda N., Hayashi S., Hattori K. Analysis of the effect of tyramine and norepinephrine in isolated canine cerebral and mesenteric arteries. J Pharmacol Exp Ther. 1978 May;205(2):382–391. [PubMed] [Google Scholar]
- Venning M. G., de la Lande I. S. Effects of uptake and surface of entry on the responses of the rat caudal artery to noradrenaline, adrenaline and methoxamine. Blood Vessels. 1984;21(4):149–155. doi: 10.1159/000158507. [DOI] [PubMed] [Google Scholar]
- Wibo M., Duong A. T., Godfraind T. Subcellular location of semicarbazide-sensitive amine oxidase in rat aorta. Eur J Biochem. 1980 Nov;112(1):87–94. doi: 10.1111/j.1432-1033.1980.tb04990.x. [DOI] [PubMed] [Google Scholar]


