Abstract
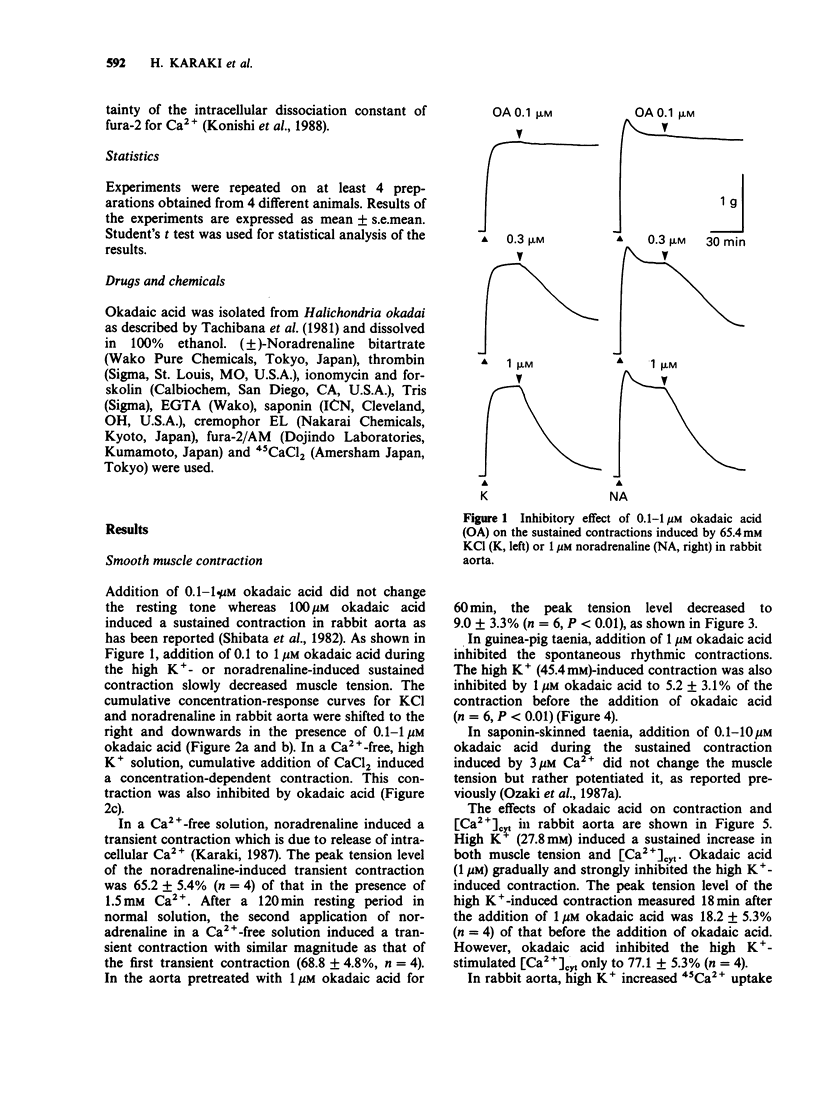
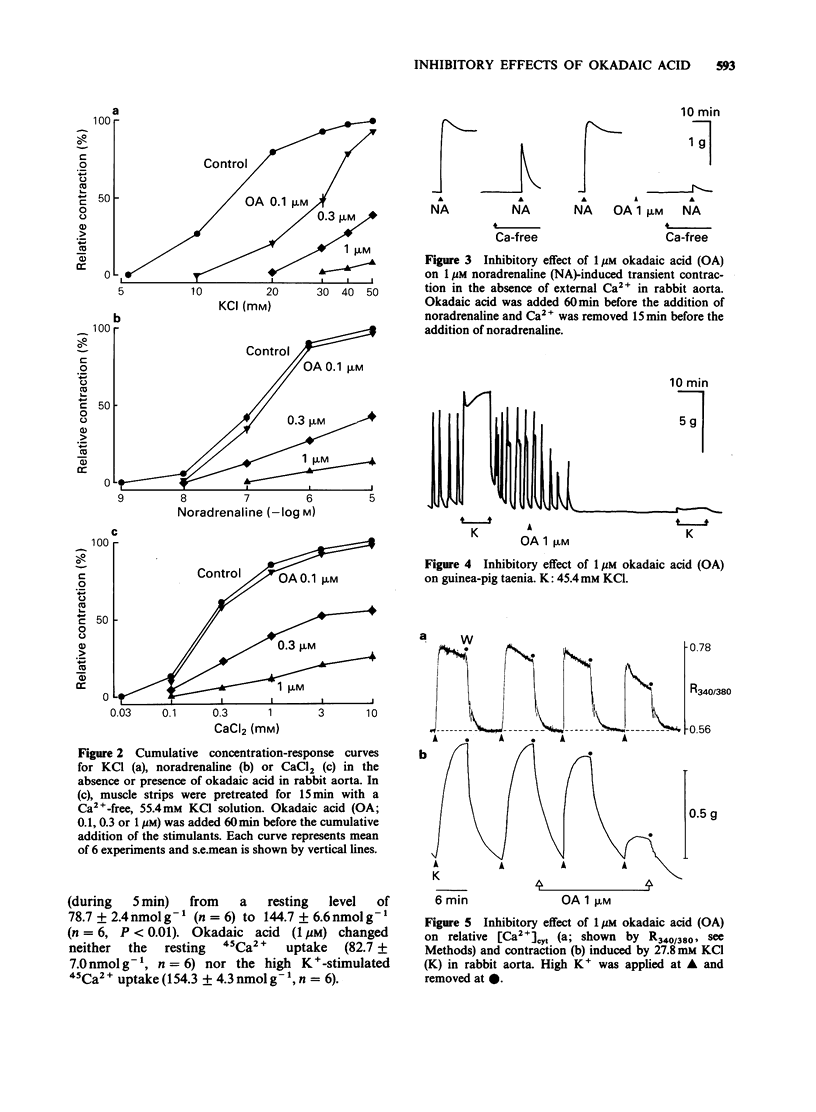
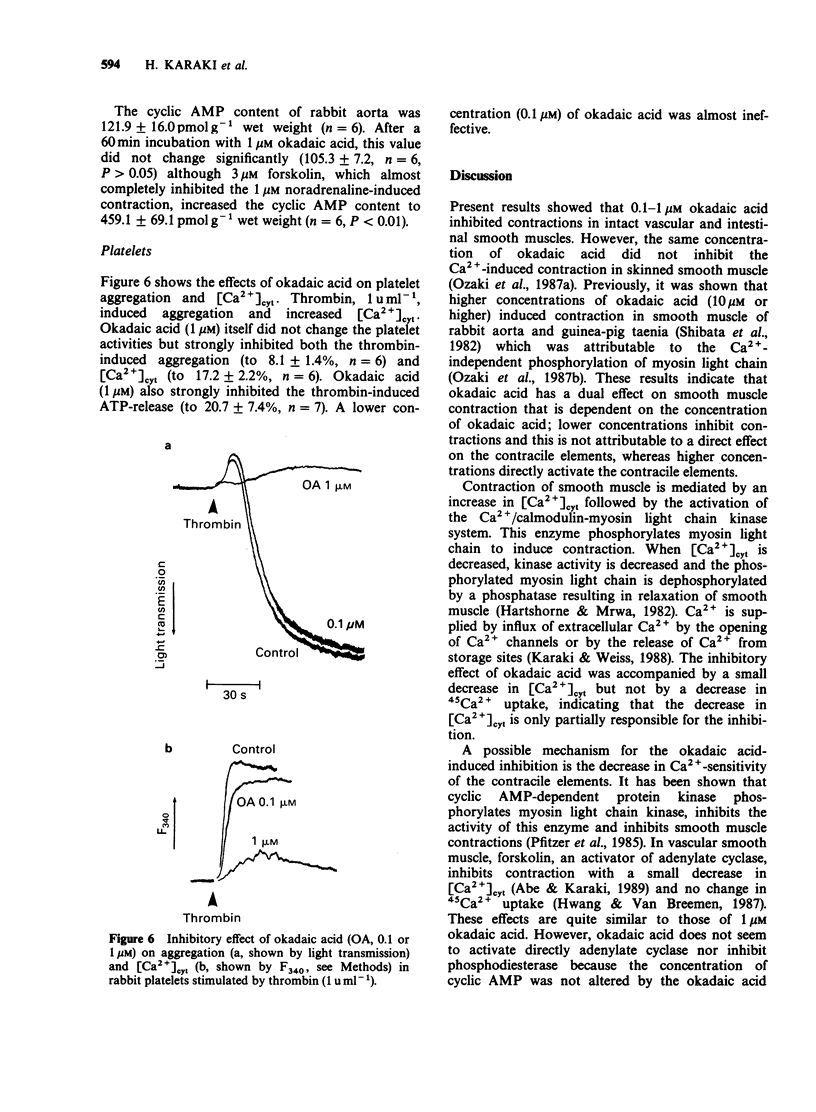
1. Effects of okadaic acid, a toxin isolated from marine sponges, on smooth muscle contraction and platelet activation were examined. 2. Contractions in rabbit aorta induced by high concentrations of K+ and noradrenaline were inhibited by 0.1-1 microM okadaic acid in a concentration-dependent manner. Spontaneous rhythmic contractions as well as high K+-induced contraction in guinea-pig taenia caeci were also inhibited by 1 microM okadaic acid. 3. High K+-induced contraction in rabbit aorta was accompanied by increased Ca2+ influx measured with 45Ca2+ and increased cytosolic Ca2+ [( Ca2+]cyt) measured with fura-2-Ca2+ fluorescence. Okadaic acid inhibited the contraction without inhibiting Ca2+ influx and produced only a small decrease in [Ca2+]cyt. 4. In a saponin-skinned taenia, Ca2+-induced contraction was not inhibited but rather potentiated by okadaic acid. 5. Okadaic acid, 1 microM, inhibited aggregation, ATP release and increased in [Ca2+]cyt induced by thrombin in washed rabbit platelets. Okadaic acid itself did not change the platelet activities. 6. Okadaic acid did not change the cyclic AMP content of rabbit aorta although the inhibitory effects of okadaic acid were similar to those of cyclic AMP. 7. Although the mechanism of the inhibitory effect of okadaic acid was not clarified in the present experiments, it is suggested that okadaic acid acts by inhibiting protein phosphatases resulting in an indirect activation of cyclic AMP-dependent protein phosphorylation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe A., Karaki H. Effect of forskolin on cytosolic Ca++ level and contraction in vascular smooth muscle. J Pharmacol Exp Ther. 1989 Jun;249(3):895–900. [PubMed] [Google Scholar]
- Abe A., Karaki H. Inhibitory effects of forskolin on vascular smooth muscle of rabbit aorta. Jpn J Pharmacol. 1988 Mar;46(3):293–301. doi: 10.1254/jjp.46.293. [DOI] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hartshorne D. J., Mrwa U. Regulation of smooth muscle actomyosin. Blood Vessels. 1982;19(1):1–18. doi: 10.1159/000158369. [DOI] [PubMed] [Google Scholar]
- Hwang K. S., Van Breemen C. Effect of dB-c-AMP and forskolin on the 45Ca influx, net Ca uptake and tension in rabbit aortic smooth muscle. Eur J Pharmacol. 1987 Feb 10;134(2):155–162. doi: 10.1016/0014-2999(87)90161-0. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989 Mar 31;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Karaki H., Sato K., Ozaki H., Murakami K. Effects of sodium nitroprusside on cytosolic calcium level in vascular smooth muscle. Eur J Pharmacol. 1988 Nov 1;156(2):259–266. doi: 10.1016/0014-2999(88)90329-9. [DOI] [PubMed] [Google Scholar]
- Karaki H., Urakawa N. Possible role of endogenous catecholamines in the contractions induced in rabbit aorta by ouabain, sodium depletion and potassium depletion. Eur J Pharmacol. 1977 May 1;43(1):65–72. doi: 10.1016/0014-2999(77)90161-3. [DOI] [PubMed] [Google Scholar]
- Karaki H. Use of tension measurements to delineate the mode of action of vasodilators. J Pharmacol Methods. 1987 Aug;18(1):1–21. doi: 10.1016/0160-5402(87)90013-1. [DOI] [PubMed] [Google Scholar]
- Karaki H., Weiss G. B. Alterations in high and low affinity binding of 45Ca in rabbit aortic smooth muscle by norepinephrine and potassium after exposure to lanthanum and low temperature. J Pharmacol Exp Ther. 1979 Oct;211(1):86–92. [PubMed] [Google Scholar]
- Karaki H., Weiss G. B. Calcium release in smooth muscle. Life Sci. 1988;42(2):111–122. doi: 10.1016/0024-3205(88)90674-1. [DOI] [PubMed] [Google Scholar]
- Konishi M., Olson A., Hollingworth S., Baylor S. M. Myoplasmic binding of fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys J. 1988 Dec;54(6):1089–1104. doi: 10.1016/S0006-3495(88)83045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Ishihara H., Kohama K., Nonomura Y., Shibata S., Karaki H. Calcium-independent phosphorylation of smooth muscle myosin light chain by okadaic acid isolated from black sponge (Halichondria okadai). J Pharmacol Exp Ther. 1987 Dec;243(3):1167–1173. [PubMed] [Google Scholar]
- Ozaki H., Kohama K., Nonomura Y., Shibata S., Karaki H. Direct activation by okadaic acid of the contractile elements in the smooth muscle of guinea-pig taenia coli. Naunyn Schmiedebergs Arch Pharmacol. 1987 Mar;335(3):356–358. doi: 10.1007/BF00172811. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Sato K., Satoh T., Karaki H. Simultaneous recordings of calcium signals and mechanical activity using fluorescent dye fura 2 in isolated strips of vascular smooth muscle. Jpn J Pharmacol. 1987 Nov;45(3):429–433. doi: 10.1254/jjp.45.429. [DOI] [PubMed] [Google Scholar]
- Pfitzer G., Merkel L., Rüegg J. C., Hofmann F. Cyclic GMP-dependent protein kinase relaxes skinned fibers from guinea pig taenia coli but not from chicken gizzard. Pflugers Arch. 1986 Jul;407(1):87–91. doi: 10.1007/BF00580726. [DOI] [PubMed] [Google Scholar]
- Pfitzer G., Rüegg J. C., Zimmer M., Hofmann F. Relaxation of skinned coronary arteries depends on the relative concentrations of Ca2+, calmodulin and active cAMP-dependent protein kinase. Pflugers Arch. 1985 Sep;405(1):70–76. doi: 10.1007/BF00591100. [DOI] [PubMed] [Google Scholar]
- Pollock W. K., Rink T. J., Irvine R. F. Liberation of [3H]arachidonic acid and changes in cytosolic free calcium in fura-2-loaded human platelets stimulated by ionomycin and collagen. Biochem J. 1986 May 1;235(3):869–877. doi: 10.1042/bj2350869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Changes in cytosolic calcium level in vascular smooth muscle strip measured simultaneously with contraction using fluorescent calcium indicator fura 2. J Pharmacol Exp Ther. 1988 Jul;246(1):294–300. [PubMed] [Google Scholar]
- Shibata S., Ishida Y., Kitano H., Ohizumi Y., Habon J., Tsukitani Y., Kikuchi H. Contractile effects of okadaic acid, a novel ionophore-like substance from black sponge, on isolated smooth muscles under the condition of Ca deficiency. J Pharmacol Exp Ther. 1982 Oct;223(1):135–143. [PubMed] [Google Scholar]


