Abstract
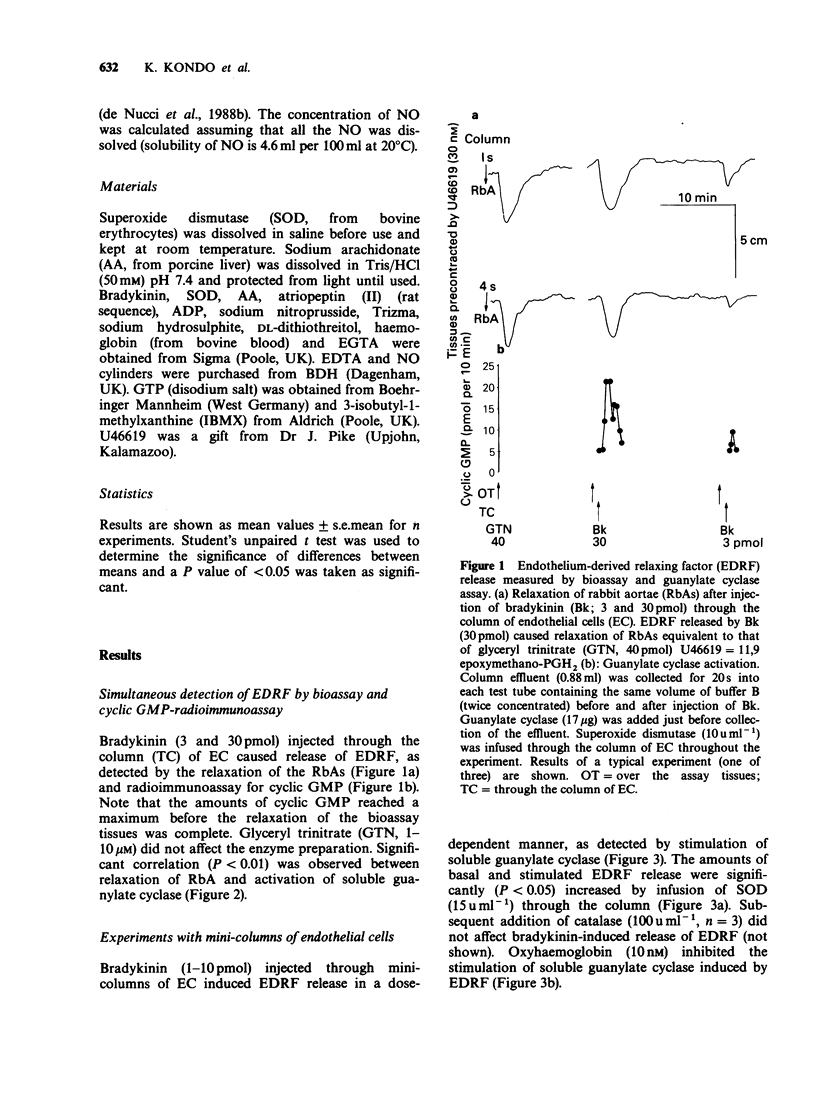
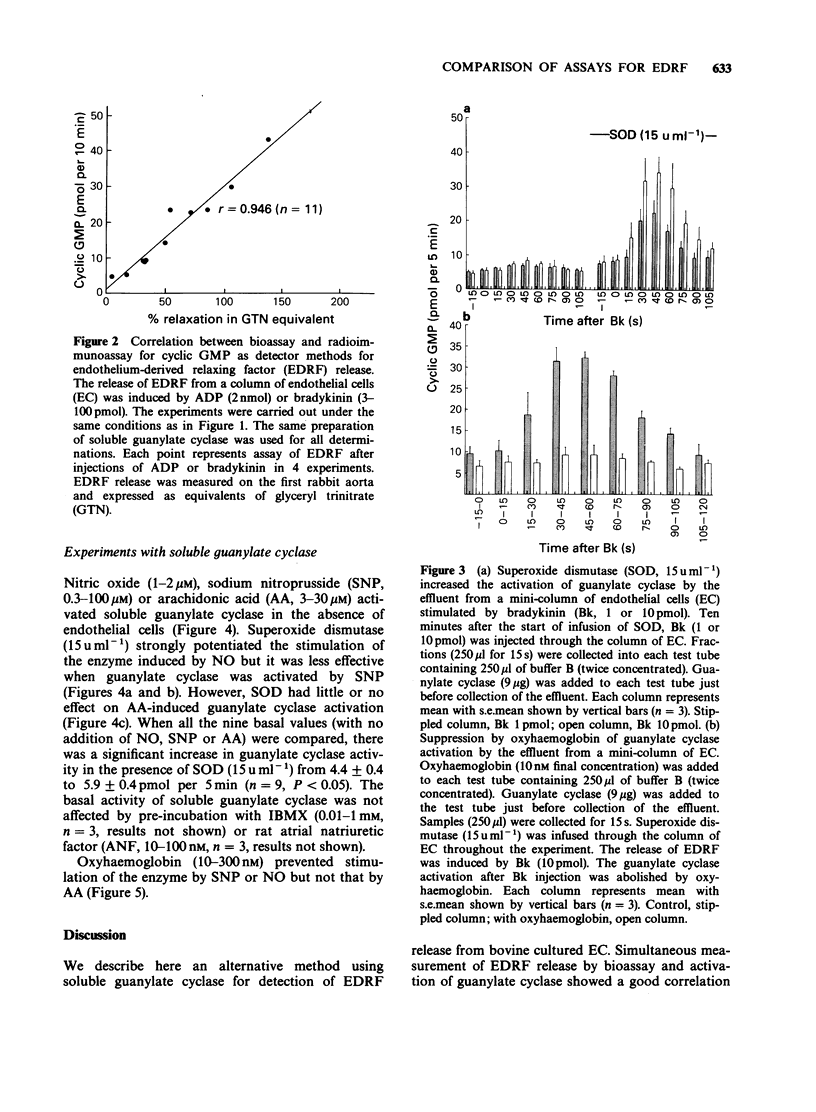
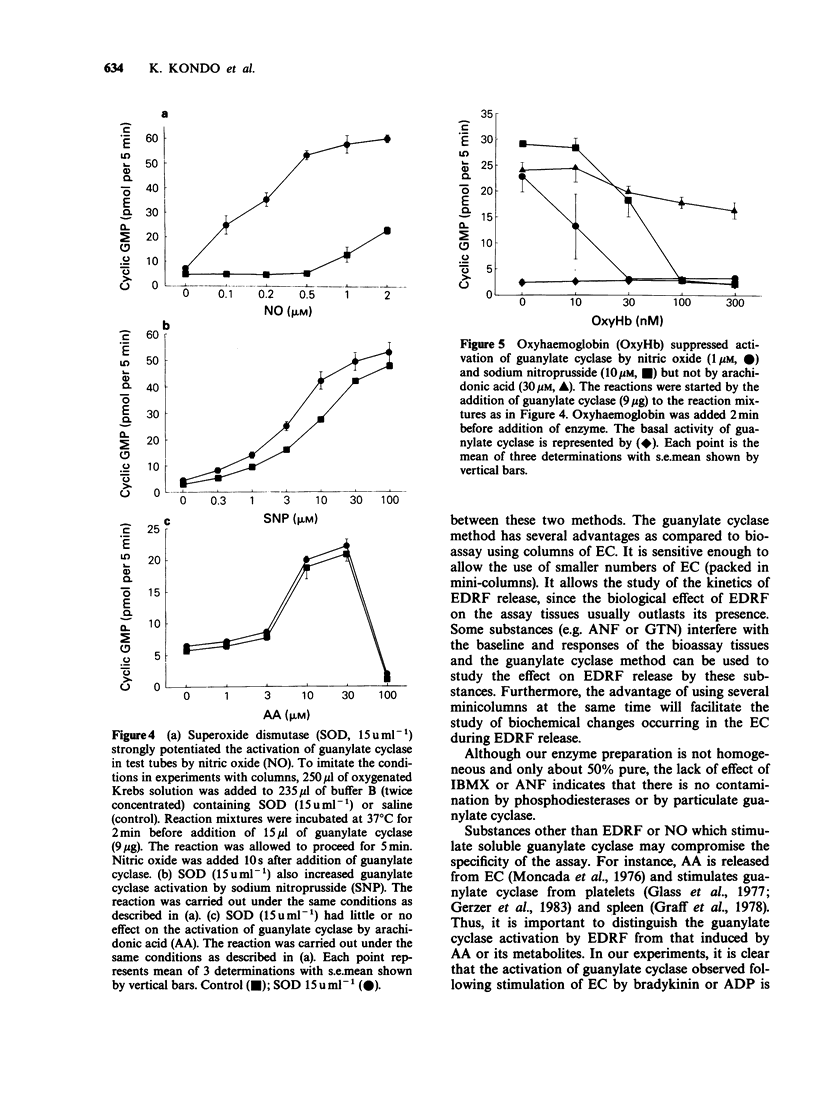
1. Endothelium-derived relaxing factor (EDRF) released by cultured endothelial cells (EC) from bovine aortae was measured by bioassay using pre-contracted strips of rabbit aorta and by radioimmunoassay of guanosine 3':5'-cyclic monophosphate (cyclic GMP) produced by stimulation of bovine lung soluble guanylate cyclase. 2. Bradykinin (Bk, 3 and 30 pmol) injected through a column of EC caused release of EDRF as detected by bioassay and increased cyclic GMP concentrations. Superoxide dismutase (SOD, 15 u ml-1) increased the amount of EDRF detected by the activation of soluble guanylate cyclase. 3. In the absence of endothelial cells, nitric oxide (NO, 1-2 microM), arachidonic acid (AA, 3-30 microM) or sodium nitroprusside (SNP, 1-100 microM) stimulated guanylate cyclase. Superoxide dismutase strongly increased the stimulation of guanylate cyclase induced by NO, but had little effect on the stimulation induced by SNP and no effect on the stimulation induced by AA. 4. Oxyhaemoglobin (10-300 microM) abolished the stimulation of guanylate cyclase by EDRF, NO or SNP but was much less effective as an inhibitor of AA-induced stimulation of guanylate cyclase. 5. These results demonstrate that measurement of guanylate cyclase stimulation by radioimmunoassay is a viable method for detecting EDRF release, especially useful when the drugs used interfere with bioassay tissues.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cocks T. M., Angus J. A., Campbell J. H., Campbell G. R. Release and properties of endothelium-derived relaxing factor (EDRF) from endothelial cells in culture. J Cell Physiol. 1985 Jun;123(3):310–320. doi: 10.1002/jcp.1041230304. [DOI] [PubMed] [Google Scholar]
- Doyle M. P., Hoekstra J. W. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J Inorg Biochem. 1981 Jul;14(4):351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gerzer R., Hamet P., Ross A. H., Lawson J. A., Hardman J. G. Calcium-induced release from platelet membranes of fatty acids that modulate soluble guanylate cyclase. J Pharmacol Exp Ther. 1983 Jul;226(1):180–186. [PubMed] [Google Scholar]
- Gerzer R., Hofmann F., Schultz G. Purification of a soluble, sodium-nitroprusside-stimulated guanylate cyclase from bovine lung. Eur J Biochem. 1981 Jun 1;116(3):479–486. doi: 10.1111/j.1432-1033.1981.tb05361.x. [DOI] [PubMed] [Google Scholar]
- Glass D. B., Frey W., 2nd, Carr D. W., Goldberg N. D. Stimulation of human platelet guanylate cyclase by fatty acids. J Biol Chem. 1977 Feb 25;252(4):1279–1285. [PubMed] [Google Scholar]
- Graff G., Stephenson J. H., Glass D. B., Haddox M. K., Goldberg N. D. Activation of soluble splenic cell guanylate cyclase by prostaglandin endoperoxides and fatty acid hydroperoxides. J Biol Chem. 1978 Nov 10;253(21):7662–7676. [PubMed] [Google Scholar]
- Gryglewski R. J., Moncada S., Palmer R. M. Bioassay of prostacyclin and endothelium-derived relaxing factor (EDRF) from porcine aortic endothelial cells. Br J Pharmacol. 1986 Apr;87(4):685–694. doi: 10.1111/j.1476-5381.1986.tb14586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Böhme E., Busse R. Stimulation of soluble guanylate cyclase by endothelium-derived relaxing factor from cultured endothelial cells. Eur J Pharmacol. 1987 Mar 17;135(2):247–250. doi: 10.1016/0014-2999(87)90620-0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Endothelium-dependent and nitrovasodilator-induced relaxation of vascular smooth muscle: role of cyclic GMP. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(4-5):281–296. [PubMed] [Google Scholar]
- Warner T. D., de Nucci G., Vane J. R. Comparison of the survival of endothelium-derived relaxing factor and nitric oxide within the isolated perfused mesenteric arterial bed of the rat. Br J Pharmacol. 1989 Jul;97(3):777–782. doi: 10.1111/j.1476-5381.1989.tb12016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nucci G., Gryglewski R. J., Warner T. D., Vane J. R. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2334–2338. doi: 10.1073/pnas.85.7.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]


