Abstract
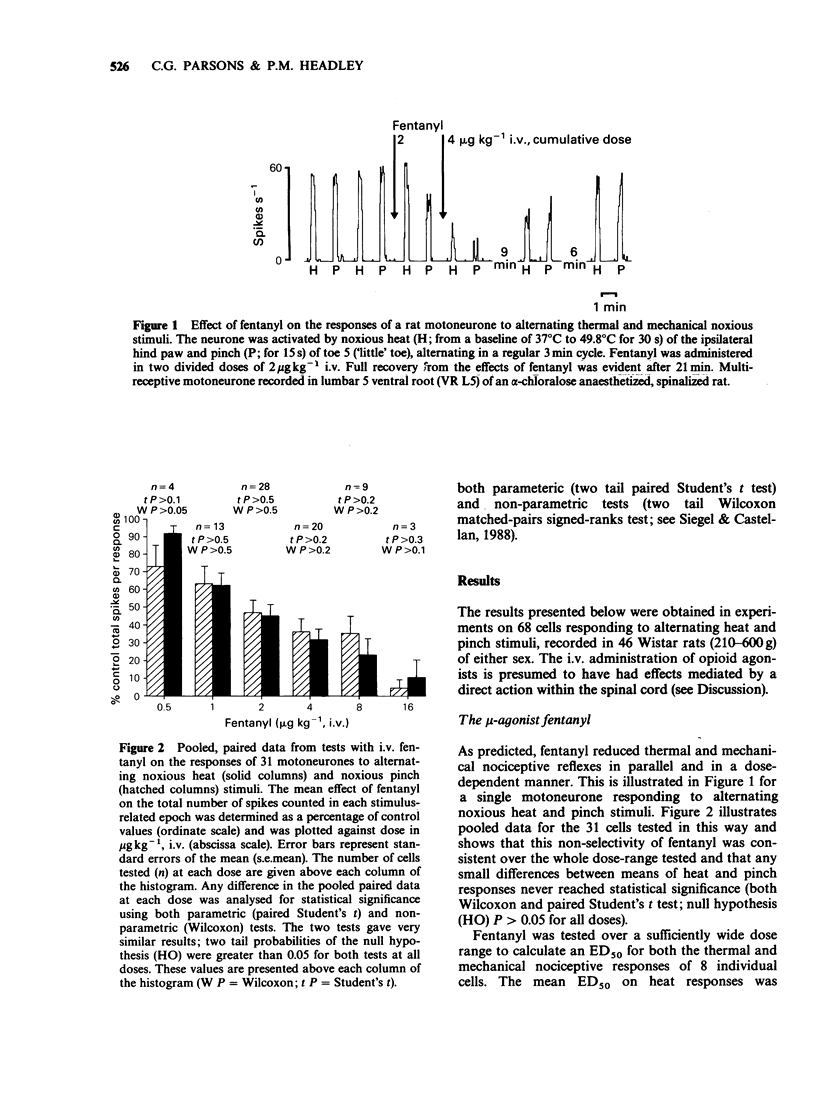
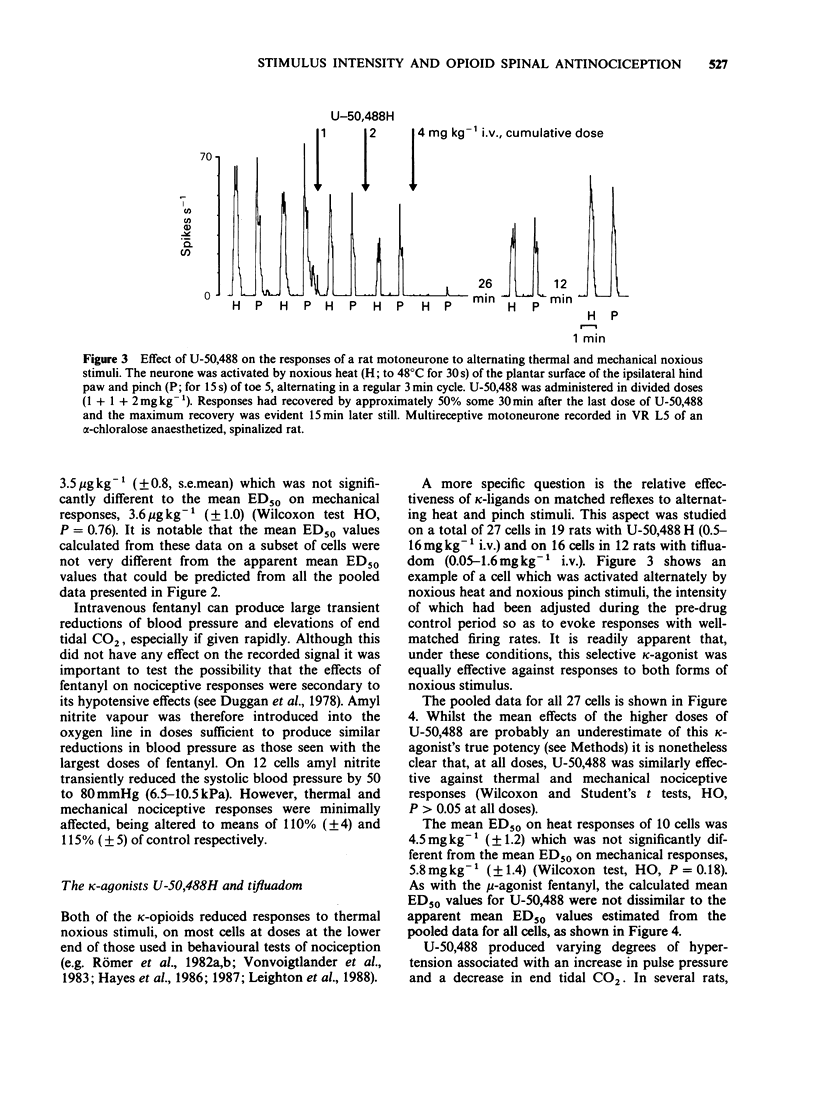
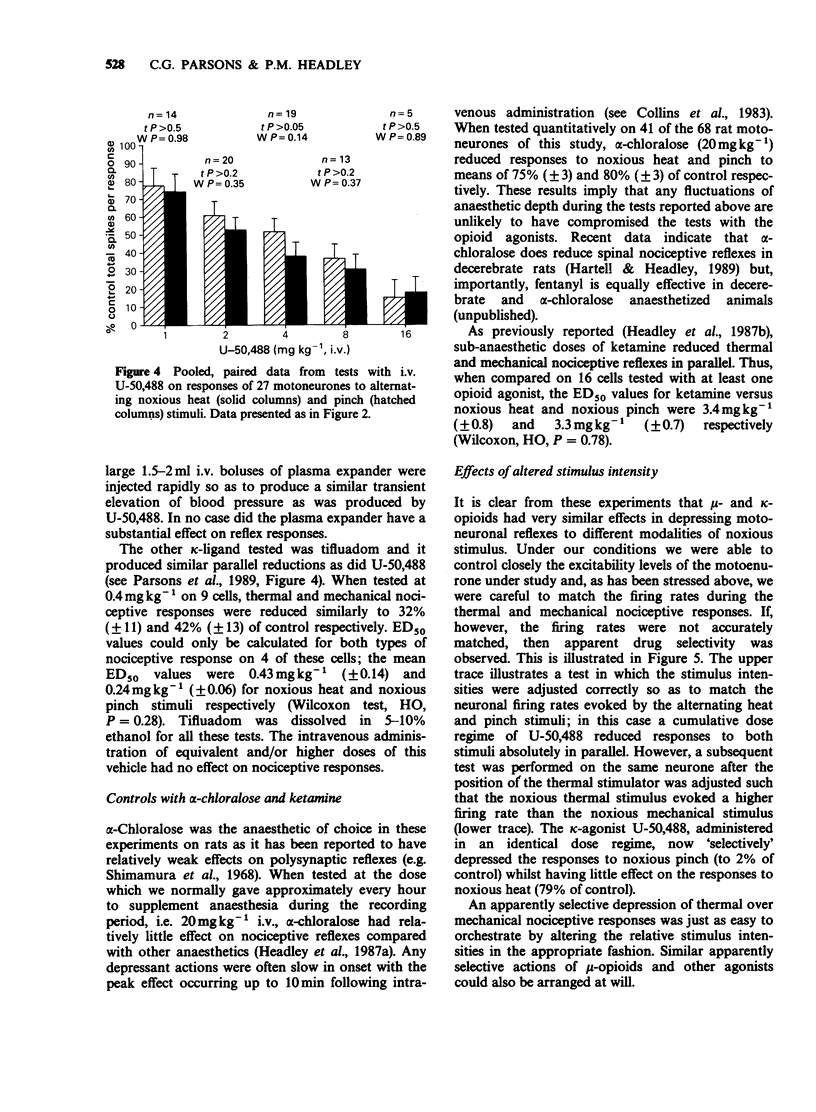
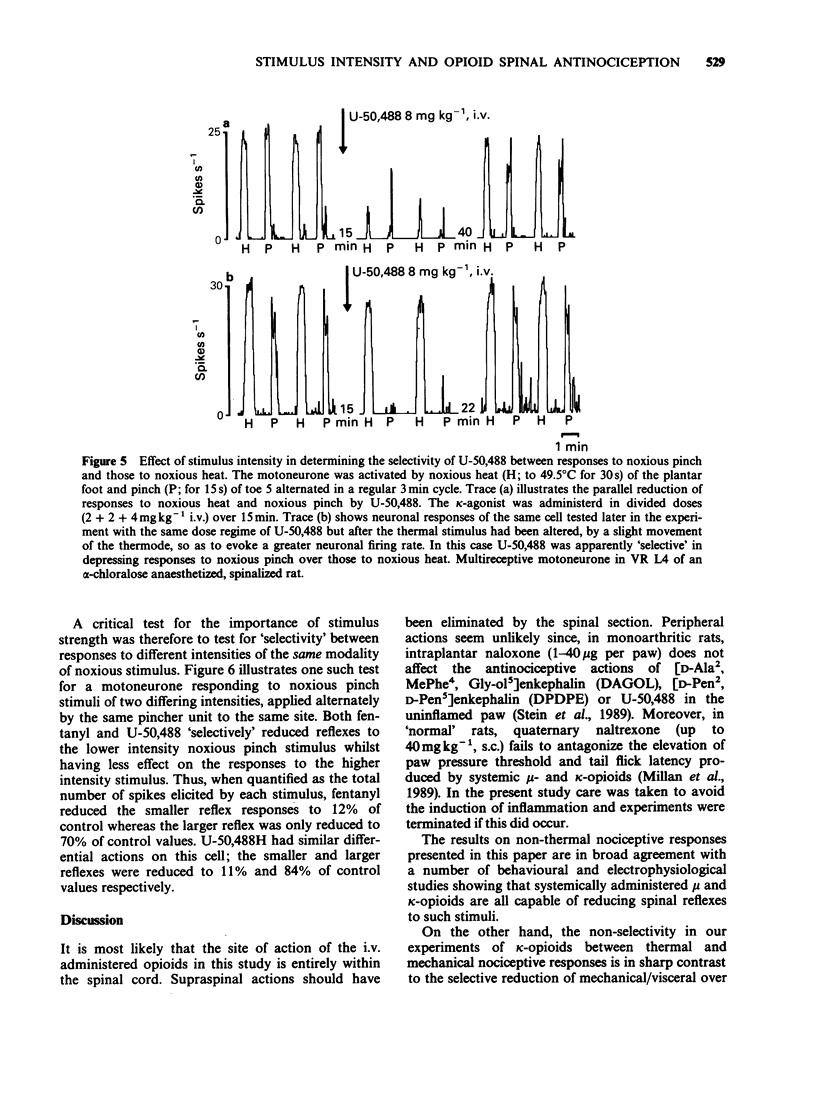
1. In electrophysiological experiments in spinalized rats, mu- and kappa-opioids were tested intravenously on the responses of single motoneurones to electronically controlled, alternating noxious heat and noxious pinch stimuli. The effects of mu- and kappa-opioids were compared with those of the general anaesthetic alpha-chloralose and the dissociative anaesthetic/PCP ligand ketamine. 2. The kappa-opioids U-50,488 (0.5-16 mgkg-1 i.v.) and tifluadom (0.05-1.6 mgkg-1 i.v.) had very similar actions to the mu-opioid fentanyl (0.5-16 micrograms kg-1 i.v.). Thus all three agonists reduced thermal and mechanical nociceptive reflexes in parallel and in a dose-dependent manner, but only so long as neuronal responses to the alternating stimuli elicited similar excitability levels in the neurone under study. Ketamine (0.5-16 mgkg-1 i.v.) had similar actions to the opioids whereas alpha-chloralose (20 mgkg-1 i.v.) had very little effect on neuronal responsiveness. 3. Apparently 'selective' depressions by both mu- and kappa-opioids could be orchestrated by a deliberate mismatch of the intensities of alternating noxious heat and pinch stimuli; as measured by neuronal firing rate, the weaker of the responses to either type of stimulus was invariably reduced to a greater degree. 4. Similar 'selectivity' could be demonstrated for both mu- and kappa-ligands when the weaker and stronger responses were of the same modality, being applied by the same pincher device but with alternating applied force. 5. It is concluded that the 'selective' spinal actions of kappa-opioids seen in non-thermal over thermal behavioural models of nociception is likely to be related to the relative intensities, rather than the modalities, of the noxious stimuli used. The validity of the interpretation of results obtained in such behavioural studies is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant R. M., Olley J. E., Tyers M. B. Antinociceptive actions of morphine and buprenorphine given intrathecally in the conscious rat. Br J Pharmacol. 1983 Apr;78(4):659–663. doi: 10.1111/j.1476-5381.1983.tb09417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard W. P. [3H]Tifluadom binding in guinea-pig brain membranes. Eur J Pharmacol. 1984 Jan 27;97(3-4):337–338. doi: 10.1016/0014-2999(84)90473-4. [DOI] [PubMed] [Google Scholar]
- Collins J. G., Kawahara M., Homma E., Kitahata L. M. Alpha-chloralose suppression of neuronal activity. Life Sci. 1983 Jun 27;32(26):2995–2999. doi: 10.1016/0024-3205(83)90651-3. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Griersmith B. T., Headley P. M., Maher J. B. The need to control skin temperature when using radiant heat in tests of analgesia. Exp Neurol. 1978 Sep 1;61(2):471–478. doi: 10.1016/0014-4886(78)90262-5. [DOI] [PubMed] [Google Scholar]
- Han J. S., Xie C. W. Dynorphin: potent analgesic effect in spinal cord of the rat. Life Sci. 1982 Oct 18;31(16-17):1781–1784. doi: 10.1016/0024-3205(82)90209-0. [DOI] [PubMed] [Google Scholar]
- Hayes A. G., Sheehan M. J., Tyers M. B. Determination of the receptor selectivity of opioid agonists in the guinea-pig ileum and mouse vas deferens by use of beta-funaltrexamine. Br J Pharmacol. 1985 Dec;86(4):899–904. doi: 10.1111/j.1476-5381.1985.tb11112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A. G., Sheehan M. J., Tyers M. B. Differential sensitivity of models of antinociception in the rat, mouse and guinea-pig to mu- and kappa-opioid receptor agonists. Br J Pharmacol. 1987 Aug;91(4):823–832. doi: 10.1111/j.1476-5381.1987.tb11281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A. G., Skingle M., Tyers M. B. Reversal by beta-funaltrexamine of the antinociceptive effect of opioid agonists in the rat. Br J Pharmacol. 1986 Aug;88(4):867–872. doi: 10.1111/j.1476-5381.1986.tb16260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley P. M., Parsons C. G., West D. C. Comparison of mu, kappa and sigma preferring agonists for effects on spinal nociceptive and other responses in rats. Neuropeptides. 1984 Dec;5(1-3):249–252. doi: 10.1016/0143-4179(84)90074-x. [DOI] [PubMed] [Google Scholar]
- Headley P. M., Parsons C. G., West D. C. The role of N-methylaspartate receptors in mediating responses of rat and cat spinal neurones to defined sensory stimuli. J Physiol. 1987 Apr;385:169–188. doi: 10.1113/jphysiol.1987.sp016490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman B. H., Goldstein A. Antinociception and paralysis induced by intrathecal dynorphin A. J Pharmacol Exp Ther. 1985 Jan;232(1):27–32. [PubMed] [Google Scholar]
- Kaneko T., Nakazawa T., Ikeda M., Yamatsu K., Iwama T., Wada T., Satoh M., Takagi H. Sites of analgesic action of dynorphin. Life Sci. 1983;33 (Suppl 1):661–664. doi: 10.1016/0024-3205(83)90589-1. [DOI] [PubMed] [Google Scholar]
- Kawakita K., Funakoshi M. A quantitative study on the tail flick test in the rat. Physiol Behav. 1987;39(2):235–240. doi: 10.1016/0031-9384(87)90015-1. [DOI] [PubMed] [Google Scholar]
- Leander J. D. Further study of kappa opioids on increased urination. J Pharmacol Exp Ther. 1983 Oct;227(1):35–41. [PubMed] [Google Scholar]
- Leighton G. E., Rodriguez R. E., Hill R. G., Hughes J. kappa-Opioid agonists produce antinociception after i.v. and i.c.v. but not intrathecal administration in the rat. Br J Pharmacol. 1988 Mar;93(3):553–560. doi: 10.1111/j.1476-5381.1988.tb10310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn B., Carpenter S. E. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982 Apr 22;238(1):29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- Magnan J., Paterson S. J., Tavani A., Kosterlitz H. W. The binding spectrum of narcotic analgesic drugs with different agonist and antagonist properties. Naunyn Schmiedebergs Arch Pharmacol. 1982 Jun;319(3):197–205. doi: 10.1007/BF00495865. [DOI] [PubMed] [Google Scholar]
- Martin W. R., Eades C. G., Thompson J. A., Huppler R. E., Gilbert P. E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976 Jun;197(3):517–532. [PubMed] [Google Scholar]
- Millan M. J. Multiple opioid systems and pain. Pain. 1986 Dec;27(3):303–347. doi: 10.1016/0304-3959(86)90158-2. [DOI] [PubMed] [Google Scholar]
- Parsons C. G., Headley P. M. On the selectivity of intravenous mu- and kappa-opioids between nociceptive and non-nociceptive reflexes in the spinalized rat. Br J Pharmacol. 1989 Oct;98(2):544–551. doi: 10.1111/j.1476-5381.1989.tb12628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons C. G., West D. C., Headley P. M. Similar actions of kappa and mu agonists on spinal nociceptive reflexes in rats and their reversibility by naloxone. NIDA Res Monogr. 1986;75:461–464. [PubMed] [Google Scholar]
- Parsons C. G., West D. C., Headley P. M. Spinal antinociceptive actions and naloxone reversibility of intravenous mu- and kappa-opioids in spinalized rats: potency mismatch with values reported for spinal administration. Br J Pharmacol. 1989 Oct;98(2):533–543. doi: 10.1111/j.1476-5381.1989.tb12627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piercey M. F., Lahti R. A., Schroeder L. A., Einspahr F. J., Barsuhn C. U-50488H, a pure kappa receptor agonist with spinal analgesic loci in the mouse. Life Sci. 1982 Sep 20;31(12-13):1197–1200. doi: 10.1016/0024-3205(82)90341-1. [DOI] [PubMed] [Google Scholar]
- Przewłocki R., Shearman G. T., Herz A. Mixed opioid/nonopioid effects of dynorphin and dynorphin related peptides after their intrathecal injection in rats. Neuropeptides. 1983 Jan;3(3):233–240. doi: 10.1016/0143-4179(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Przewłocki R., Stala L., Greczek M., Shearman G. T., Przewłocka B., Herz A. Analgesic effects of mu-, delta- and kappa-opiate agonists and, in particular, dynorphin at the spinal level. Life Sci. 1983;33 (Suppl 1):649–652. doi: 10.1016/0024-3205(83)90586-6. [DOI] [PubMed] [Google Scholar]
- Römer D., Büscher H. H., Hill R. C., Maurer R., Petcher T. J., Zeugner H., Benson W., Finner E., Milkowski W., Thies P. W. An opioid benzodiazepine. Nature. 1982 Aug 19;298(5876):759–760. doi: 10.1038/298759a0. [DOI] [PubMed] [Google Scholar]
- Römer D., Büscher H. H., Hill R. C., Maurer R., Petcher T. J., Zeugner H., Benson W., Finner E., Milkowski W., Thies P. W. Unexpected opioid activity in a known class of drug. Life Sci. 1982 Sep 20;31(12-13):1217–1220. doi: 10.1016/0024-3205(82)90346-0. [DOI] [PubMed] [Google Scholar]
- Schmauss C. Spinal kappa-opioid receptor-mediated antinociception is stimulus-specific. Eur J Pharmacol. 1987 Jun 4;137(2-3):197–205. doi: 10.1016/0014-2999(87)90223-8. [DOI] [PubMed] [Google Scholar]
- Schmauss C., Yaksh T. L. In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. J Pharmacol Exp Ther. 1984 Jan;228(1):1–12. [PubMed] [Google Scholar]
- Schmauss C., Yaksh T. L., Shimohigashi Y., Harty G., Jensen T., Rodbard D. Differential association of spinal mu, delta and kappa opioid receptors with cutaneous thermal and visceral chemical nociceptive stimuli in the rat. Life Sci. 1983;33 (Suppl 1):653–656. doi: 10.1016/0024-3205(83)90587-8. [DOI] [PubMed] [Google Scholar]
- Shimamura M., Yamauchi T., Aoki M. Effects of chloralose anesthesia on spinal reflexes. Jpn J Physiol. 1968 Dec 15;18(6):788–797. doi: 10.2170/jjphysiol.18.788. [DOI] [PubMed] [Google Scholar]
- Spampinato S., Candeletti S. Characterization of dynorphin A-induced antinociception at spinal level. Eur J Pharmacol. 1985 Mar 26;110(1):21–30. doi: 10.1016/0014-2999(85)90024-x. [DOI] [PubMed] [Google Scholar]
- Tyers M. B. A classification of opiate receptors that mediate antinociception in animals. Br J Pharmacol. 1980 Jul;69(3):503–512. doi: 10.1111/j.1476-5381.1980.tb07041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton N., Sewell R. D., Spencer P. S. Differentiation of potent mu and kappa-opiate agonists using heat and pressure antinociceptive profiles and combined potency analysis. Eur J Pharmacol. 1982 Mar 26;78(4):421–429. doi: 10.1016/0014-2999(82)90484-8. [DOI] [PubMed] [Google Scholar]
- Vonvoigtlander P. F., Lahti R. A., Ludens J. H. U-50,488: a selective and structurally novel non-Mu (kappa) opioid agonist. J Pharmacol Exp Ther. 1983 Jan;224(1):7–12. [PubMed] [Google Scholar]
- Ward S. J., Takemori A. E. Relative involvement of mu, kappa and delta receptor mechanisms in opiate-mediated antinociception in mice. J Pharmacol Exp Ther. 1983 Mar;224(3):525–530. [PubMed] [Google Scholar]
- Yaksh T. L., Noueihed R. The physiology and pharmacology of spinal opiates. Annu Rev Pharmacol Toxicol. 1985;25:433–462. doi: 10.1146/annurev.pa.25.040185.002245. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Rudy T. A. Analgesia mediated by a direct spinal action of narcotics. Science. 1976 Jun 25;192(4246):1357–1358. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]


