Abstract
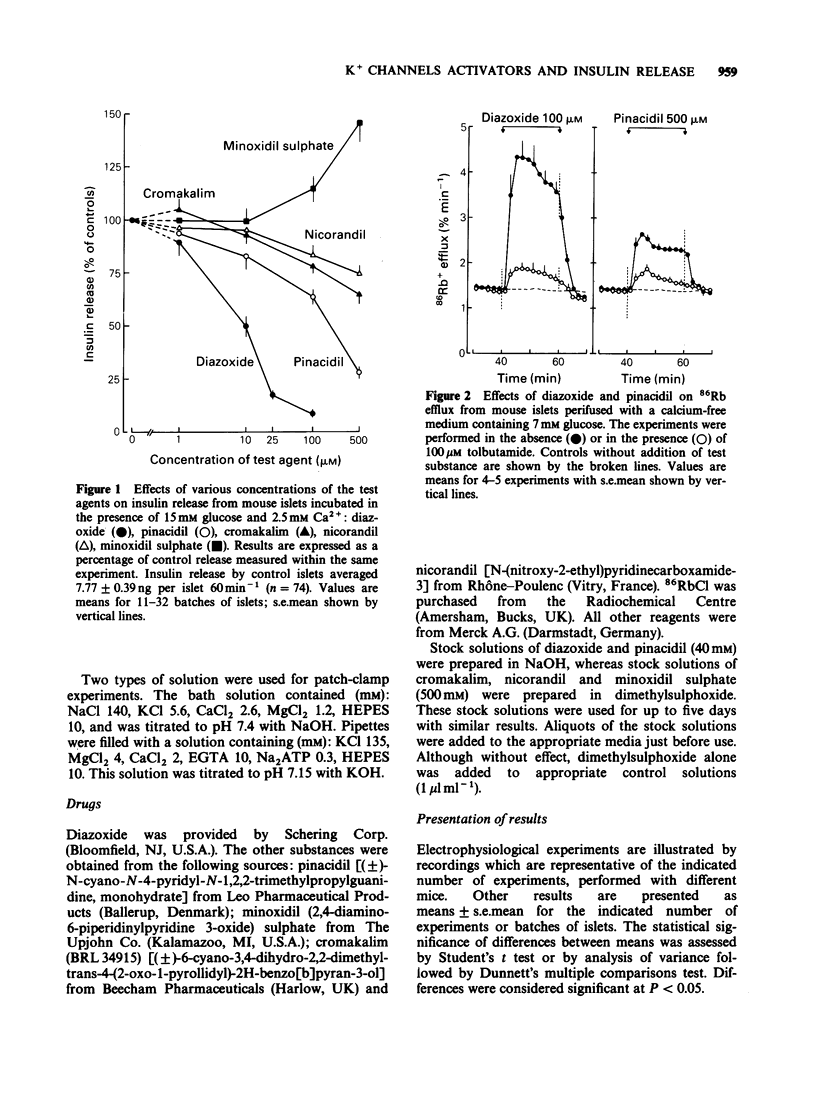
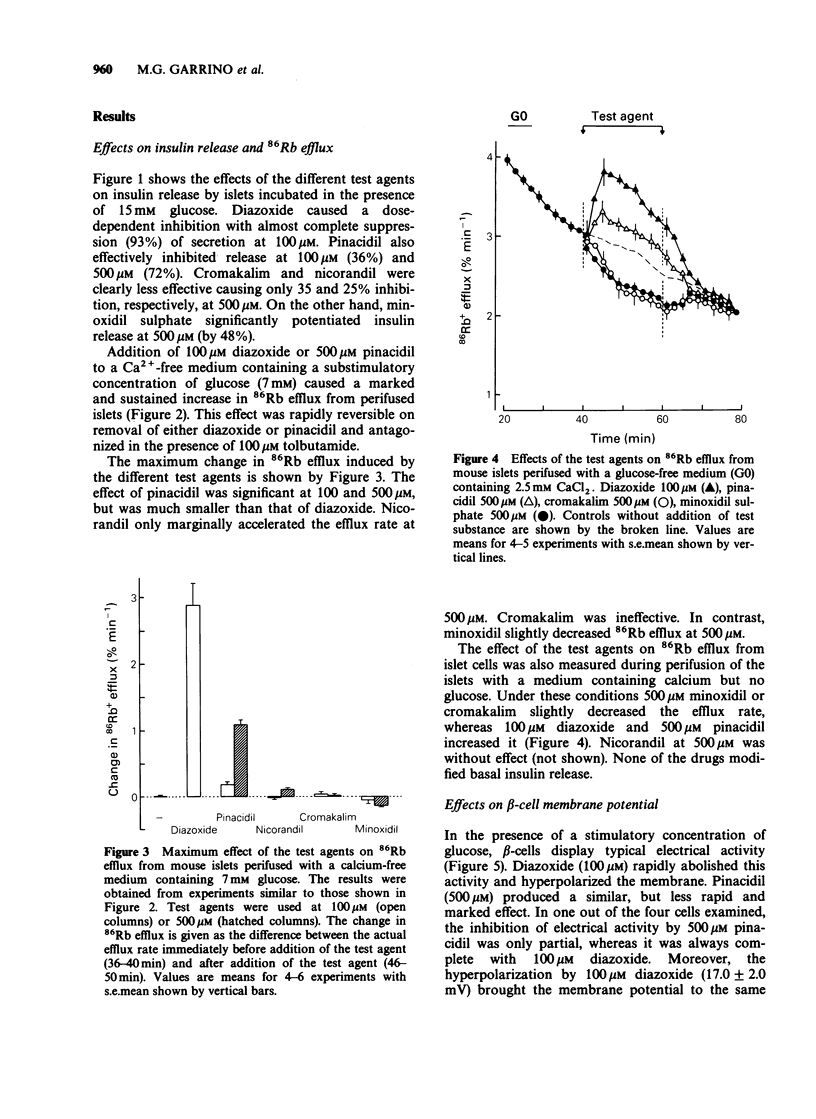
1 The vasodilator and antihypertensive properties of pinacidil, cromakalim (BRL 34915), nicorandil and minoxidil sulphate may be due, at least in part, to their ability to open K+ channels in vascular smooth muscles. In this study, mouse pancreatic islets were used to determine whether these drugs affect insulin release by acting on K+ channels of beta-cells. Their effects were compared to those of diazoxide. 2 Diazoxide caused a dose-dependent inhibition of insulin release by islets incubated with 15 mM glucose (93% at 100 microM). Pinacidil inhibited release by 36 and 72% at 100 and 500 microM, respectively. Cromakalim and nicorandil were less effective (35 and 25% inhibition at 500 microM). Minoxidil sulphate increased insulin release at 500 microM. 3 In the presence of 7 mM glucose and in the absence of Ca2+ (to avoid activation of Ca2+-dependent K+ channels), 86Rb efflux from islet cells was increased by 100-500 microM pinacidil and 500 microM nicorandil, which were, however, less potent than diazoxide. Cromakalim was ineffective, whereas 500 microM minoxidil sulphate decreased the efflux rate. In the absence of glucose and presence of Ca2+, 500 microM cromakalim and minoxidil sulphate inhibited 86Rb efflux. 4 Like diazoxide, pinacidil (500 microM) abolished glucose-induced electrical activity in beta-cells and hyperpolarized the membrane. 5 ATP-sensitive K+ currents were studied in single beta-cells by the whole cell patch-clamp technique. Pinacidil increased the current less than did diazoxide. In contrast, cromakalim and minoxidil sulphate decreased K+-currents whilst nicorandil was without effect.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft F. M., Ashcroft S. J., Harrison D. E. Properties of single potassium channels modulated by glucose in rat pancreatic beta-cells. J Physiol. 1988 Jun;400:501–527. doi: 10.1113/jphysiol.1988.sp017134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Harrison D. E., Ashcroft S. J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. 1984 Nov 29-Dec 5Nature. 312(5993):446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Atwater I., Ribalet B., Rojas E. Cyclic changes in potential and resistance of the beta-cell membrane induced by glucose in islets of Langerhans from mouse. J Physiol. 1978 May;278:117–139. doi: 10.1113/jphysiol.1978.sp012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belles B., Hescheler J., Trube G. Changes of membrane currents in cardiac cells induced by long whole-cell recordings and tolbutamide. Pflugers Arch. 1987 Aug;409(6):582–588. doi: 10.1007/BF00584657. [DOI] [PubMed] [Google Scholar]
- Bray K. M., Newgreen D. T., Small R. C., Southerton J. S., Taylor S. G., Weir S. W., Weston A. H. Evidence that the mechanism of the inhibitory action of pinacidil in rat and guinea-pig smooth muscle differs from that of glyceryl trinitrate. Br J Pharmacol. 1987 Jun;91(2):421–429. doi: 10.1111/j.1476-5381.1987.tb10297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Cook N. S., Quast U., Hof R. P., Baumlin Y., Pally C. Similarities in the mechanism of action of two new vasodilator drugs: pinacidil and BRL 34915. J Cardiovasc Pharmacol. 1988 Jan;11(1):90–99. doi: 10.1097/00005344-198801000-00014. [DOI] [PubMed] [Google Scholar]
- Cook N. S. The pharmacology of potassium channels and their therapeutic potential. Trends Pharmacol Sci. 1988 Jan;9(1):21–28. doi: 10.1016/0165-6147(88)90238-6. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Illot M. C., Peterson O. H. Interaction of diazoxide, tolbutamide and ATP4- on nucleotide-dependent K+ channels in an insulin-secreting cell line. J Membr Biol. 1987;99(3):215–224. doi: 10.1007/BF01995702. [DOI] [PubMed] [Google Scholar]
- Escande D., Thuringer D., Leguern S., Cavero I. The potassium channel opener cromakalim (BRL 34915) activates ATP-dependent K+ channels in isolated cardiac myocytes. Biochem Biophys Res Commun. 1988 Jul 29;154(2):620–625. doi: 10.1016/0006-291x(88)90184-2. [DOI] [PubMed] [Google Scholar]
- Garrino M. G., Henquin J. C. Highly potent and stereoselective effects of the benzoic acid derivative AZ-DF 265 on pancreatic beta-cells. Br J Pharmacol. 1988 Jan;93(1):61–68. doi: 10.1111/j.1476-5381.1988.tb11405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrino M. G., Schmeer W., Nenquin M., Meissner H. P., Henquin J. C. Mechanism of the stimulation of insulin release in vitro by HB 699, a benzoic acid derivative similar to the non-sulphonylurea moiety of glibenclamide. Diabetologia. 1985 Sep;28(9):697–703. doi: 10.1007/BF00291979. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Weston A. H. Cromakalim, nicorandil and pinacidil: novel drugs which open potassium channels in smooth muscle. Gen Pharmacol. 1989;20(1):1–9. doi: 10.1016/0306-3623(89)90052-9. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Charles S., Nenquin M., Mathot F., Tamagawa T. Diazoxide and D600 inhibition of insulin release. Distinct mechanisms explain the specificity for different stimuli. Diabetes. 1982 Sep;31(9):776–783. doi: 10.2337/diab.31.9.776. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. D-glucose inhibits potassium efflux from pancreatic islet cells. Nature. 1978 Jan 19;271(5642):271–273. doi: 10.1038/271271a0. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Lambert A. E. Cobalt inhibition of insulin secretion and calcium uptake by isolated rat islets. Am J Physiol. 1975 Jun;228(6):1669–1677. doi: 10.1152/ajplegacy.1975.228.6.1669. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Opposite effects of tolbutamide and diazoxide on 86Rb+ fluxes and membrane potential in pancreatic B cells. Biochem Pharmacol. 1982 Apr 1;31(7):1407–1415. doi: 10.1016/0006-2952(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Regulation of insulin release by ionic and electrical events in B cells. Horm Res. 1987;27(3):168–178. doi: 10.1159/000180806. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Tolbutamide stimulation and inhibition of insulin release: studies of the underlying ionic mechanisms in isolated rat islets. Diabetologia. 1980;18(2):151–160. doi: 10.1007/BF00290493. [DOI] [PubMed] [Google Scholar]
- Hof D. A pulse generating and data recording system based on the microcomputer PDP 11/23. Comput Methods Programs Biomed. 1986 Dec;23(3):309–315. doi: 10.1016/0169-2607(86)90065-9. [DOI] [PubMed] [Google Scholar]
- Hollingsworth M., Amédée T., Edwards D., Mironneau J., Savineau J. P., Small R. C., Weston A. H. The relaxant action of BRL 34915 in rat uterus. Br J Pharmacol. 1987 Aug;91(4):803–813. doi: 10.1111/j.1476-5381.1987.tb11279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch-Weser J. Diazoxide. N Engl J Med. 1976 Jun 3;294(23):1271–1273. doi: 10.1056/NEJM197606032942306. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Laychock S. G. Evidence for guanosine 3',5'-monophosphate as a putative mediator of insulin secretion from isolated rat islets. Endocrinology. 1981 Apr;108(4):1197–1205. doi: 10.1210/endo-108-4-1197. [DOI] [PubMed] [Google Scholar]
- Lebrun P., Devreux V., Hermann M., Herchuelz A. Pinacidil inhibits insulin release by increasing K+ outflow from pancreatic B-cells. Eur J Pharmacol. 1988 Nov 1;156(2):283–286. doi: 10.1016/0014-2999(88)90334-2. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., Cipkus L. A., Taylor C. J. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988 Jun;245(3):751–760. [PubMed] [Google Scholar]
- Meissner H. P., Schmelz H. Membrane potential of beta-cells in pancreatic islets. Pflugers Arch. 1974;351(3):195–206. doi: 10.1007/BF00586918. [DOI] [PubMed] [Google Scholar]
- Misler S., Falke L. C., Gillis K., McDaniel M. L. A metabolite-regulated potassium channel in rat pancreatic B cells. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7119–7123. doi: 10.1073/pnas.83.18.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C. K., Arrigoni-Martelli E. Effect of a new vasodilator, pinacidil (P 1134), on potassium, noradrenaline and serotonin induced contractions in rabbit vascular tissues. Acta Pharmacol Toxicol (Copenh) 1981 Nov;49(5):427–431. doi: 10.1111/j.1600-0773.1981.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Findlay I. Electrophysiology of the pancreas. Physiol Rev. 1987 Jul;67(3):1054–1116. doi: 10.1152/physrev.1987.67.3.1054. [DOI] [PubMed] [Google Scholar]
- Plant T. D. Properties and calcium-dependent inactivation of calcium currents in cultured mouse pancreatic B-cells. J Physiol. 1988 Oct;404:731–747. doi: 10.1113/jphysiol.1988.sp017316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast U. Effect of the K+ efflux stimulating vasodilator BRL 34915 on 86Rb+ efflux and spontaneous activity in guinea-pig portal vein. Br J Pharmacol. 1987 Jul;91(3):569–578. doi: 10.1111/j.1476-5381.1987.tb11250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Glucose dependent K+-channels in pancreatic beta-cells are regulated by intracellular ATP. Pflugers Arch. 1985 Dec;405(4):305–309. doi: 10.1007/BF00595682. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Scott A. L., Zingaro G. J., Siegl P. K. BRL 34915 (cromakalim) activates ATP-sensitive K+ current in cardiac muscle. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8360–8364. doi: 10.1073/pnas.85.21.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlin J., Taljedal I. B. Glucose-induced decrease in Rb+ permeability in pancreatic beta cells. Nature. 1975 Feb 20;253(5493):635–636. doi: 10.1038/253635a0. [DOI] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Weir S. W., Weston A. H. The effects of BRL 34915 and nicorandil on electrical and mechanical activity and on 86Rb efflux in rat blood vessels. Br J Pharmacol. 1986 May;88(1):121–128. doi: 10.1111/j.1476-5381.1986.tb09478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist R. J., Heaney L. A., Wallace A. A., Baskin E. P., Stein R. B., Garcia M. L., Kaczorowski G. J. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989 Jan;248(1):149–156. [PubMed] [Google Scholar]
- Zünkler B. J., Lenzen S., Männer K., Panten U., Trube G. Concentration-dependent effects of tolbutamide, meglitinide, glipizide, glibenclamide and diazoxide on ATP-regulated K+ currents in pancreatic B-cells. Naunyn Schmiedebergs Arch Pharmacol. 1988 Feb;337(2):225–230. doi: 10.1007/BF00169252. [DOI] [PubMed] [Google Scholar]


