Abstract
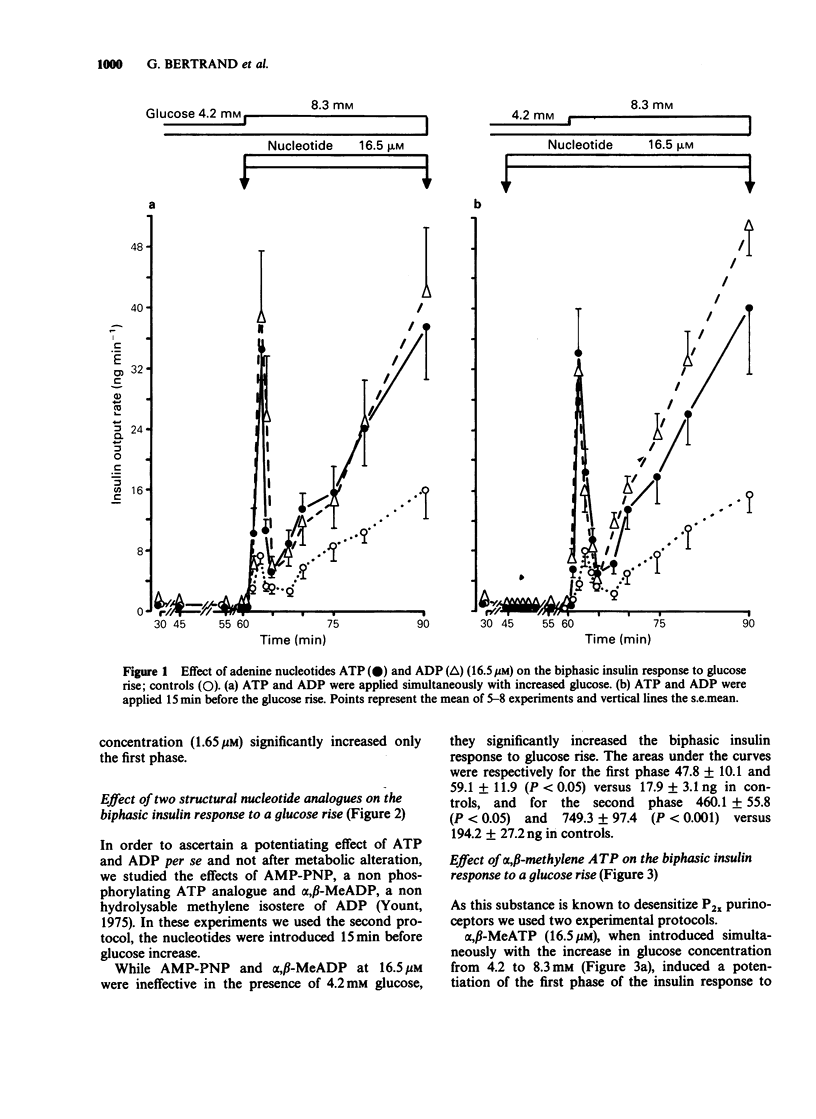
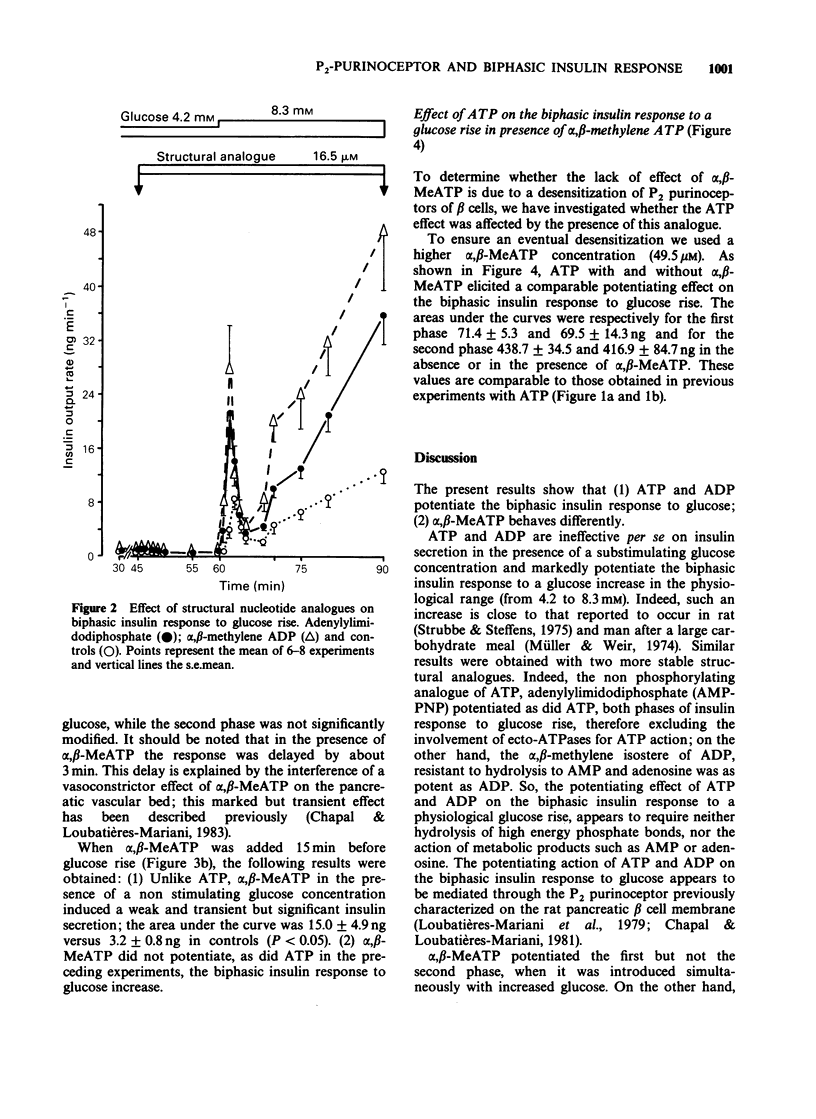
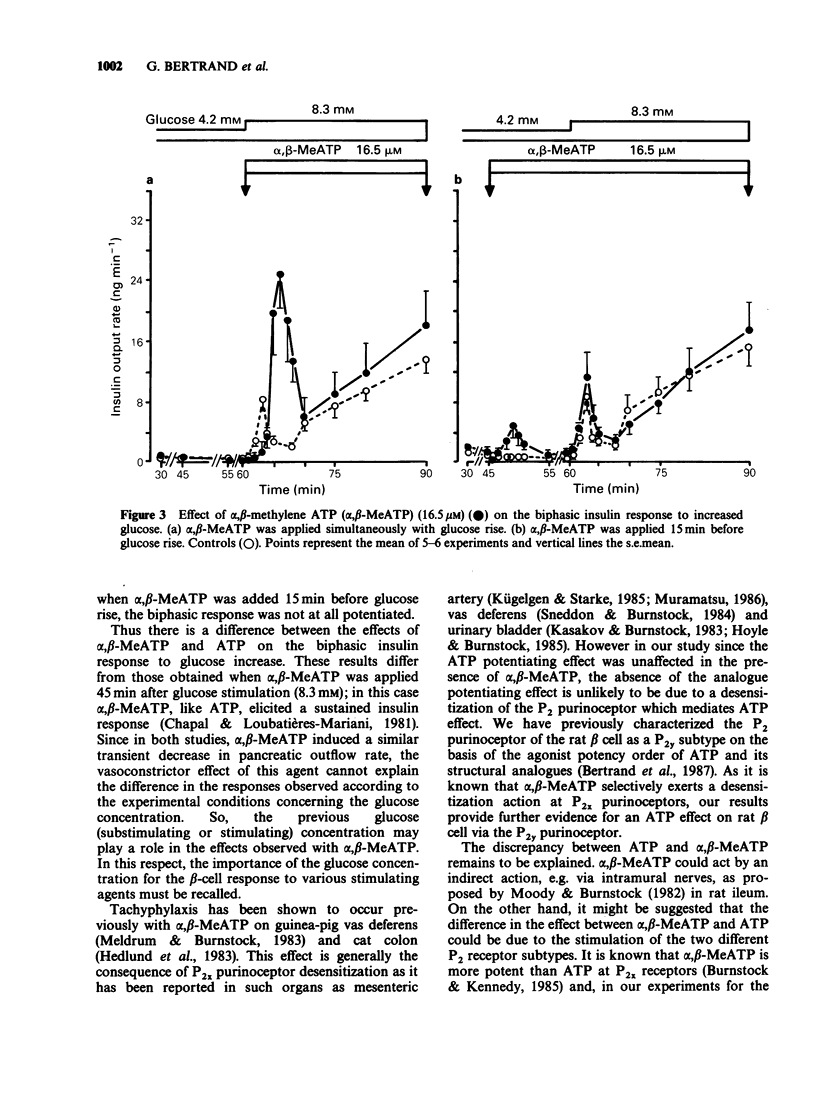
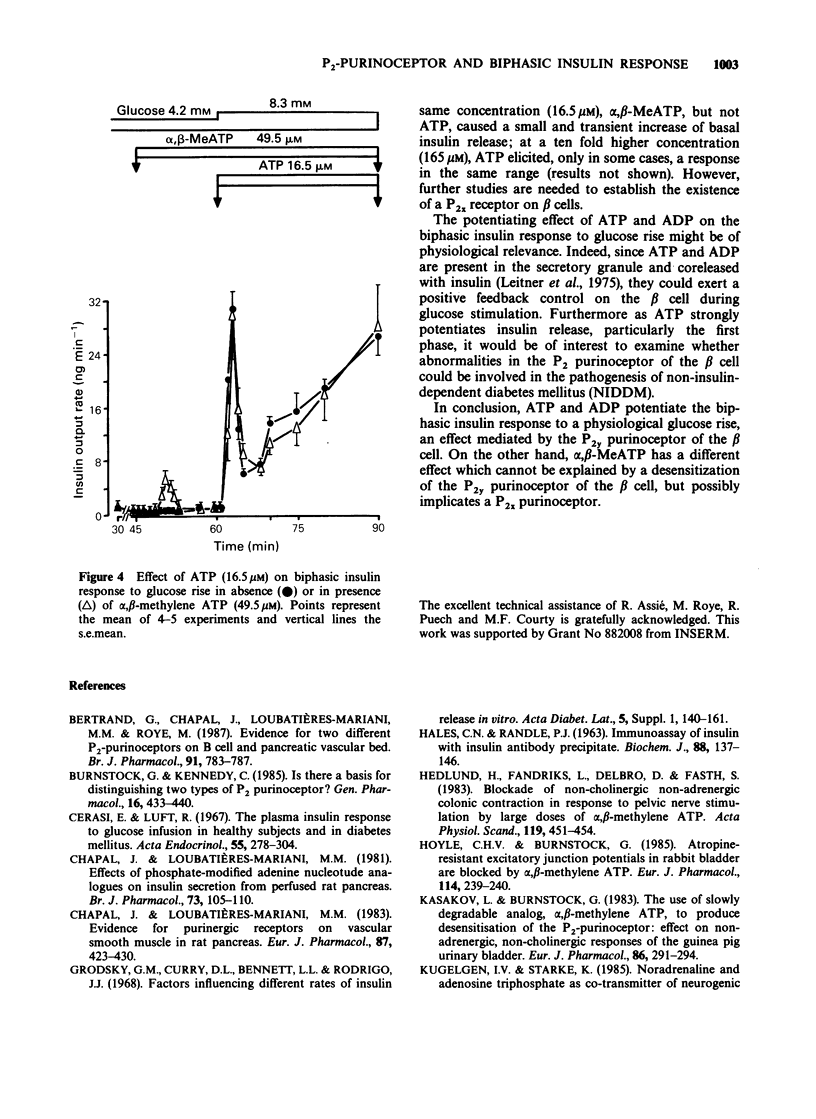
1. The effects of exogenous adenine nucleotides and structural analogues on the biphasic insulin response to an increase of glucose concentration in the physiological range (from 4.2 to 8.3 mM) were studied in the isolated perfused rat pancreas. Purinoceptor agonists were added either simultaneously or 15 min before increasing glucose. 2. ATP and ADP at 16.5 microM were ineffective per se in the presence of the non stimulatory glucose concentration (4.2 mM) but markedly potentiated the biphasic insulin response to glucose rise in both experimental protocols. 3. Two more stable analogues of ATP and ADP (adenylylimidodiphosphate and alpha, beta-methylene ADP (alpha, beta-MeADP)) at 16.5 microM behaved like the natural compounds: they were ineffective at a glucose concentration of 4.2 mM and potentiated both phases of insulin response to glucose rise. 4. alpha, beta-MeATP added simultaneously with the high glucose concentration, markedly potentiated the first phase of insulin response to glucose rise but did not potentiate the second one. When alpha, beta-MeATP infusion began 15 min before glucose rise, the biphasic response to glucose was not potentiated, in contrast to what occurred with ATP. 5. In the presence of alpha, beta-MeATP, the ATP potentiating effect was unaffected. 6. It is concluded that ATP and ADP, via activation of beta cell P2 gamma purinoceptors, potentiates the biphasic insulin response to an increase of glucose concentration. On the other hand, alpha, beta-MeATP did not behave like natural and other structural analogues of ATP and ADP: this difference appears not to be the consequence of desensitization of beta cell P2 gamma purinoceptors by alpha, beta-MeATP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertrand G., Chapal J., Loubatières-Mariani M. M., Roye M. Evidence for two different P2-purinoceptors on beta cell and pancreatic vascular bed. Br J Pharmacol. 1987 Aug;91(4):783–787. doi: 10.1111/j.1476-5381.1987.tb11276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Cerasi E., Luft R. The plasma insulin response to glucose infusion in healthy subjects and in diabetes mellitus. Acta Endocrinol (Copenh) 1967 Jun;55(2):278–304. doi: 10.1530/acta.0.0550278. [DOI] [PubMed] [Google Scholar]
- Chapal J., Loubatieres-Mariani M. M. Effects of phosphate-modified adenine nucleotide analogues on insulin secretion from perfused rat pancreas. Br J Pharmacol. 1981 May;73(1):105–110. doi: 10.1111/j.1476-5381.1981.tb16778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapal J., Loubatieres-Mariani M. M. Evidence for purinergic receptors on vascular smooth muscle in rat pancreas. Eur J Pharmacol. 1983 Mar 4;87(4):423–430. doi: 10.1016/0014-2999(83)90081-x. [DOI] [PubMed] [Google Scholar]
- Grodsky G. M., Curry D. L., Bennett L. L., Rodrigo J. J. [Factors influencing different rates of insulin release in vitro]. Acta Diabetol Lat. 1968 Oct;5 (Suppl 1):140–161. [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund H., Fändriks L., Delbro D., Fasth S. Blockade of non-cholinergic non-adrenergic colonic contraction in response to pelvic nerve stimulation by large doses of alpha, beta-methylene ATP. Acta Physiol Scand. 1983 Dec;119(4):451–454. doi: 10.1111/j.1748-1716.1983.tb07361.x. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Burnstock G. Atropine-resistant excitatory junction potentials in rabbit bladder are blocked by alpha,beta-methylene ATP. Eur J Pharmacol. 1985 Aug 15;114(2):239–240. doi: 10.1016/0014-2999(85)90635-1. [DOI] [PubMed] [Google Scholar]
- Kasakov L., Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982 Dec 24;86(2):291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Loubatieres-Mariani M. M., Chapal J., Lignon F., Valette G. Structural specificity of nucleotides for insulin secretory action from the isolated perfused rat pancreas. Eur J Pharmacol. 1979 Nov 16;59(3-4):277–286. doi: 10.1016/0014-2999(79)90291-7. [DOI] [PubMed] [Google Scholar]
- Loubatières A., Mariani M. M., Ribes G., de Malbosc H., Chapal J. Etude expérimentale d'un nouveau sulfamide hypoglycémiant particulièrement actif, le HB 419 ou glibenclamide. Diabetologia. 1969 Feb;5(1):1–10. doi: 10.1007/BF01212212. [DOI] [PubMed] [Google Scholar]
- Meldrum L. A., Burnstock G. Evidence that ATP acts as a co-transmitter with noradrenaline in sympathetic nerves supplying the guinea-pig vas deferens. Eur J Pharmacol. 1983 Aug 19;92(1-2):161–163. doi: 10.1016/0014-2999(83)90126-7. [DOI] [PubMed] [Google Scholar]
- Moody C. J., Burnstock G. Evidence for the presence of P1-purinoceptors on cholinergic nerve terminals in the guinea-pig ileum. Eur J Pharmacol. 1982 Jan 8;77(1):1–9. doi: 10.1016/0014-2999(82)90527-1. [DOI] [PubMed] [Google Scholar]
- Muramatsu I. Evidence for sympathetic, purinergic transmission in the mesenteric artery of the dog. Br J Pharmacol. 1986 Mar;87(3):478–480. doi: 10.1111/j.1476-5381.1986.tb10187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984 Apr 13;100(1):85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- Strubbe J. H., Steffens A. B. Rapid insulin release after ingestion of a meal in the unanesthetized rat. Am J Physiol. 1975 Oct;229(4):1019–1022. doi: 10.1152/ajplegacy.1975.229.4.1019. [DOI] [PubMed] [Google Scholar]
- Yount R. G. ATP analogs. Adv Enzymol Relat Areas Mol Biol. 1975;43:1–56. doi: 10.1002/9780470122884.ch1. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline and adenosine triphosphate as co-transmitters of neurogenic vasoconstriction in rabbit mesenteric artery. J Physiol. 1985 Oct;367:435–455. doi: 10.1113/jphysiol.1985.sp015834. [DOI] [PMC free article] [PubMed] [Google Scholar]


