Abstract
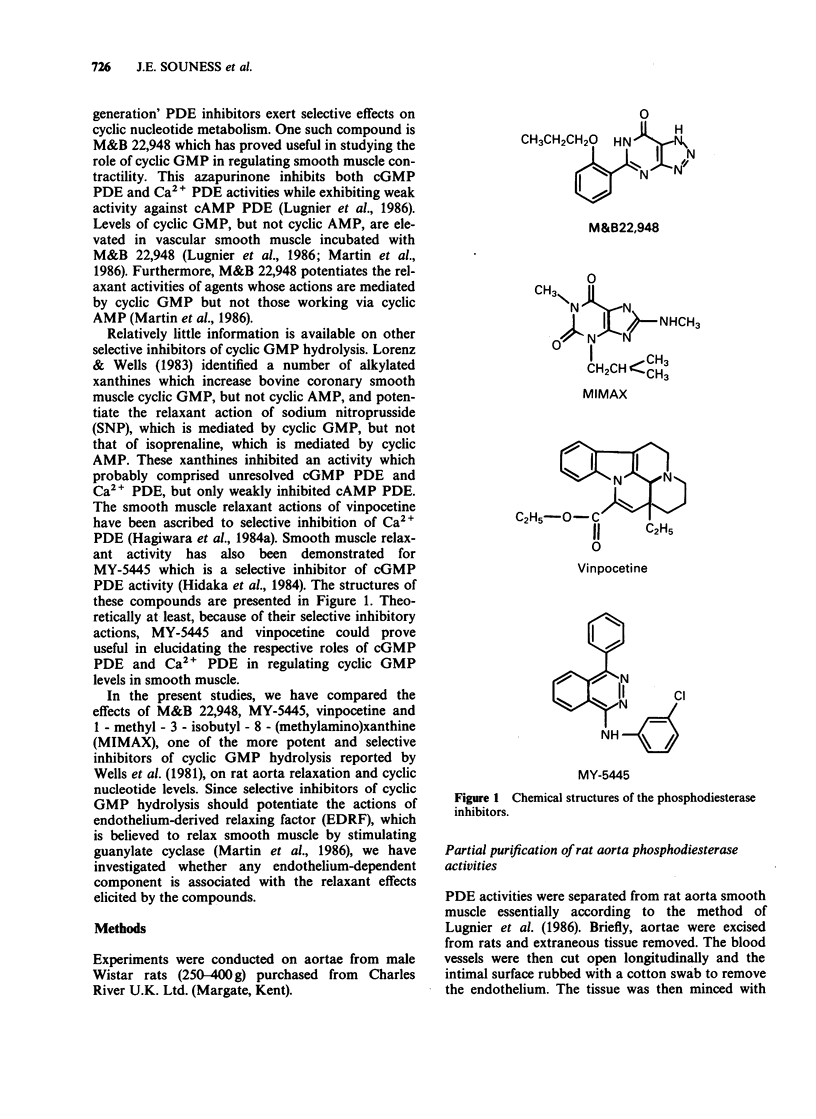
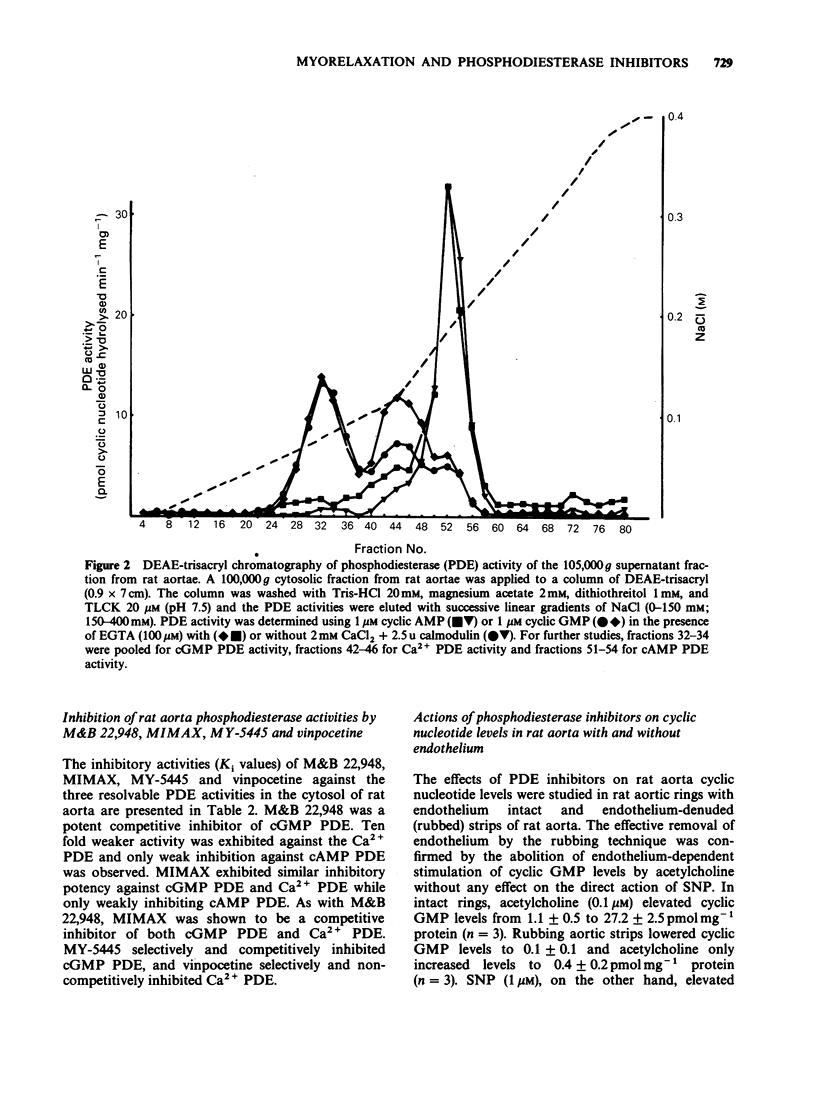
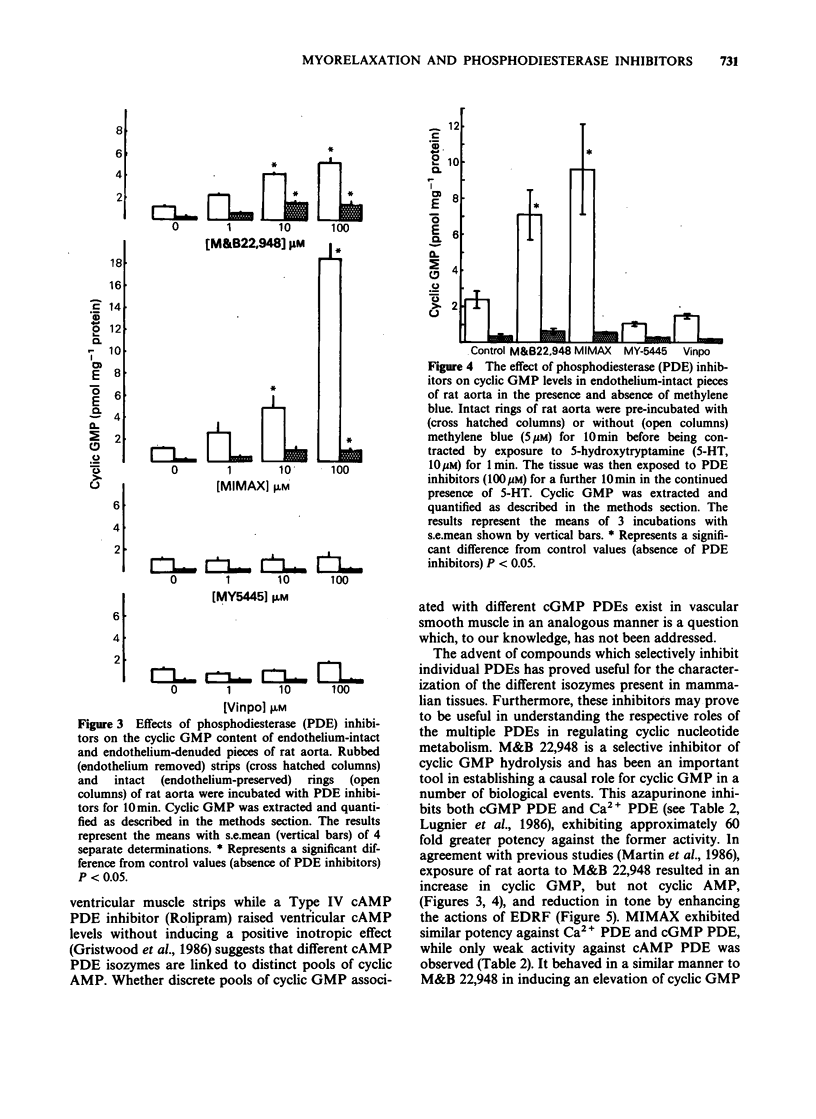
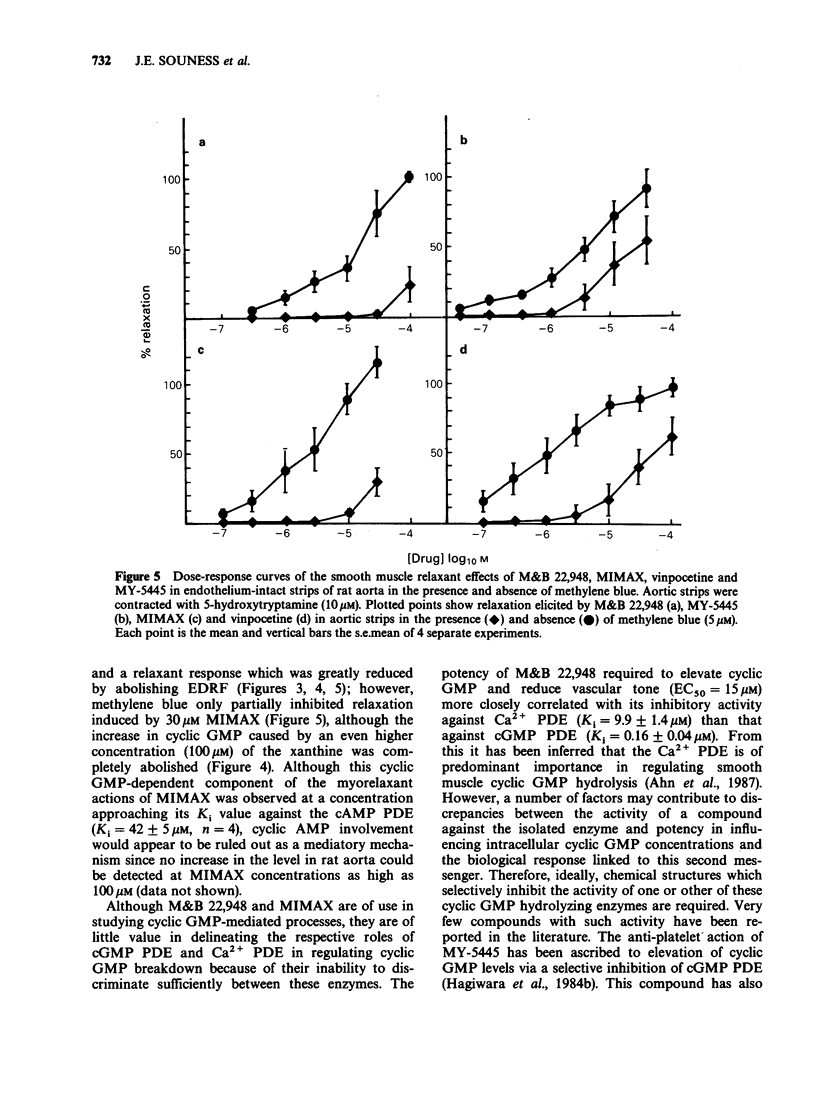
1. The mechanism by which M&B 22,948, MY-5445, vinpocetine and 1-methyl-3-isobutyl-8-(methylamino)xanthine (MIMAX), which have been described as selective cyclic GMP phosphodiesterase (PDE) inhibitors, relax rat aorta was investigated. 2. Three cyclic nucleotide PDEs were identified in the soluble fraction of rat aorta; a Ca2+-insensitive form exhibiting substrate selectivity for cyclic GMP (cGMP PDE), a Ca2+/calmodulin-stimulated form which also preferentially hydrolyzed cyclic GMP (Ca2+ PDE), and a form demonstrating substrate selectivity for cyclic AMP (cAMP PDE). 3. M&B 22,948 and MIMAX inhibited cGMP PDE (Ki = 0.16 microM and 0.43 microM, respectively) and Ca2+ PDE (Ki = 9.9 microM and 0.55 microM, respectively), but exhibited weak activity against cAMP PDE (Ki = 249 microM and 42 microM, respectively). MY-5445 selectivity inhibited cGMP PDE (Ki = 1.3 microM) and vinpocetine selectively inhibited Ca2+ PDE (Ki = 14 microM). 4. M&B 22,948 and MIMAX induced dose-dependent increases in the accumulation of cyclic GMP, but not cyclic AMP, in rat aorta pieces. These effects were greatly reduced by endothelial denudation and by methylene blue (5 microM) which blocks the actions of endothelium-derived relaxant factor. MY-5445 and vinpocetine had no effect on rat aorta cyclic GMP or cyclic AMP accumulation. 5. All four compounds caused dose-related relaxation of 5-hydroxytryptamine (10 microM) contracted, endothelium-intact rat aorta, the effects of M&B 22,948 and MIMAX being greatly reduced by methylene blue (5 microM). Methylene blue also caused 10 fold and 100 fold rightward shifts in the dose-response curves of MY-5445 and vinpocetine, respectively. 6. The results are consistent with the smooth muscle relaxant actions of M&B 22,948 and MIMAX, but not vinpocetine and MY-5445, being mediated through a mechanism involving inhibition of cyclic GMP hydrolysis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arimura H., Ikemoto Y. Action of enflurane on cholinergic transmission in identified Aplysia neurones. Br J Pharmacol. 1986 Nov;89(3):573–582. doi: 10.1111/j.1476-5381.1986.tb11158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Drummond A. H. Cyclic GMP mediates neurogenic relaxation in the bovine retractor penis muscle. Br J Pharmacol. 1984 Apr;81(4):665–674. doi: 10.1111/j.1476-5381.1984.tb16133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England P. J., Shahid M. Effects of forskolin on contractile responses and protein phosphorylation in the isolated perfused rat heart. Biochem J. 1987 Sep 15;246(3):687–695. doi: 10.1042/bj2460687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara M., Endo T., Hidaka H. Effects of vinpocetine on cyclic nucleotide metabolism in vascular smooth muscle. Biochem Pharmacol. 1984 Feb 1;33(3):453–457. doi: 10.1016/0006-2952(84)90240-5. [DOI] [PubMed] [Google Scholar]
- Hagiwara M., Endo T., Kanayama T., Hidaka H. Effect of 1-(3-chloroanilino)-4-phenylphthalazine (MY-5445), a specific inhibitor of cyclic GMP phosphodiesterase, on human platelet aggregation. J Pharmacol Exp Ther. 1984 Feb;228(2):467–471. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lorenz K. L., Wells J. N. Potentiation of the effects of sodium nitroprusside and of isoproterenol by selective phosphodiesterase inhibitors. Mol Pharmacol. 1983 Mar;23(2):424–430. [PubMed] [Google Scholar]
- Lugnier C., Schoeffter P., Le Bec A., Strouthou E., Stoclet J. C. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem Pharmacol. 1986 May 15;35(10):1743–1751. doi: 10.1016/0006-2952(86)90333-3. [DOI] [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Phosphodiesterase inhibitors induce endothelium-dependent relaxation of rat and rabbit aorta by potentiating the effects of spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):539–547. [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Michel A. D., Whiting R. L. Direct binding studies on ileal and cardiac muscarinic receptors. Br J Pharmacol. 1987 Dec;92(4):755–767. doi: 10.1111/j.1476-5381.1987.tb11379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. R., Wells J. N. Effects of isoproterenol on active force and Ca2+ X calmodulin-sensitive phosphodiesterase activity in porcine coronary artery. Biochem Pharmacol. 1987 Jun 1;36(11):1819–1824. doi: 10.1016/0006-2952(87)90244-9. [DOI] [PubMed] [Google Scholar]
- Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986 Jul;78(1):1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves M. L., Leigh B. K., England P. J. The identification of a new cyclic nucleotide phosphodiesterase activity in human and guinea-pig cardiac ventricle. Implications for the mechanism of action of selective phosphodiesterase inhibitors. Biochem J. 1987 Jan 15;241(2):535–541. doi: 10.1042/bj2410535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeffter P., Lugnier C., Demesy-Waeldele F., Stoclet J. C. Role of cyclic AMP- and cyclic GMP-phosphodiesterases in the control of cyclic nucleotide levels and smooth muscle tone in rat isolated aorta. A study with selective inhibitors. Biochem Pharmacol. 1987 Nov 15;36(22):3965–3972. doi: 10.1016/0006-2952(87)90465-5. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Terasaki W. L., Epstein P. M., Strada S. J. Assay of cyclic nucleotide phosphodiesterase and resolution of multiple molecular forms of the enzyme. Adv Cyclic Nucleotide Res. 1979;10:69–92. [PubMed] [Google Scholar]
- Weishaar R. E., Cain M. H., Bristol J. A. A new generation of phosphodiesterase inhibitors: multiple molecular forms of phosphodiesterase and the potential for drug selectivity. J Med Chem. 1985 May;28(5):537–545. doi: 10.1021/jm50001a001. [DOI] [PubMed] [Google Scholar]
- Wells J. N., Garst J. E., Kramer G. L. Inhibition of separated forms of cyclic nucleotide phosphodiesterase from pig coronary arteries by 1,3-disubstituted and 1,3,8-trisubstituted xanthines. J Med Chem. 1981 Aug;24(8):954–958. doi: 10.1021/jm00140a008. [DOI] [PubMed] [Google Scholar]


