Abstract
1. The gamma-aminobutyric acid (GABA) receptors on the somatic muscle cells of Ascaris, which mediate muscle cell hyperpolarization and relaxation, have been characterized by use of intracellular recording techniques. 2. These receptors are like mammalian GABAA-receptors in that the response is mediated by an increase conductance to chloride ions. The GABAA-mimetic, muscimol, has a relative potency of 0.40 +/- 0.02 (n = 3) compared to GABA. 3. The stereoselectivity of the GABA receptor on Ascaris is identical to that for the mammalian GABAA-receptor, as determined from the relative potency of three pairs of enantiomers of structural analogues of GABA. 4. The most potent agonist is (S)-(+)-dihydromuscimol which is 7.53 +/- 0.98 (n = 5) times more potent than GABA. 5. The Ascaris GABA receptor is not significantly blocked, at concentrations below 100 microM by the potent, competitive GABAA-receptor antagonist, SR95531. 6. The Ascaris GABA receptor does not recognise agents that are known to block the GABA gated chloride channel in mammalian preparations such as t-butylbicyclophosphorothionate (TBPS, 10 microM, n = 2) or the insecticide dieldrin (100 microM, n = 3). 7. GABAergic responses in Ascaris are not potentiated by pentobarbitone (100 microM, n = 3) or flurazepam (100 microM, n = 3). 8. The potencies of various GABA-mimetics in the Ascaris preparation have been compared with their potency at displacing GABAA-receptor binding in mammalian brain. Excluding the sulphonic acid derivatives of GABA, the correlation coefficient (r) between the potencies of compounds in the two systems is 0.74 (P less than 0.01). The significance of this correlation is discussed. 9. The pharmacology of the Ascaris GABA receptor is discussed in relation to other invertebrate systems and the mammalian subclassification of GABA receptors.
Full text
PDF

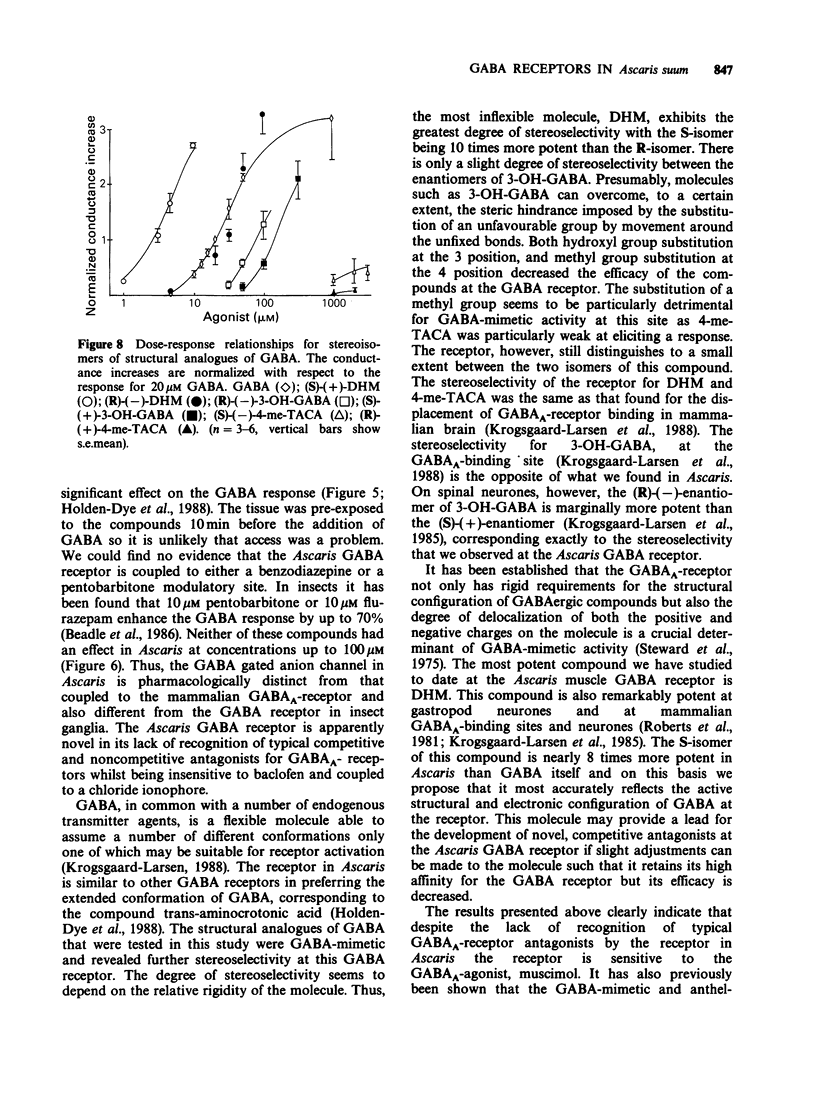


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan R. D., Dickenson H. W., Hiern B. P., Johnston G. A., Kazlauskas R. Isothiouronium compounds as gamma-aminobutyric acid agonists. Br J Pharmacol. 1986 Jun;88(2):379–387. doi: 10.1111/j.1476-5381.1986.tb10214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard M. E., Smith A. D. Secretion of acetylcholinesterase and butyrylcholinesterase from the guinea-pig isolated ileum. Br J Pharmacol. 1989 Jun;97(2):490–498. doi: 10.1111/j.1476-5381.1989.tb11977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
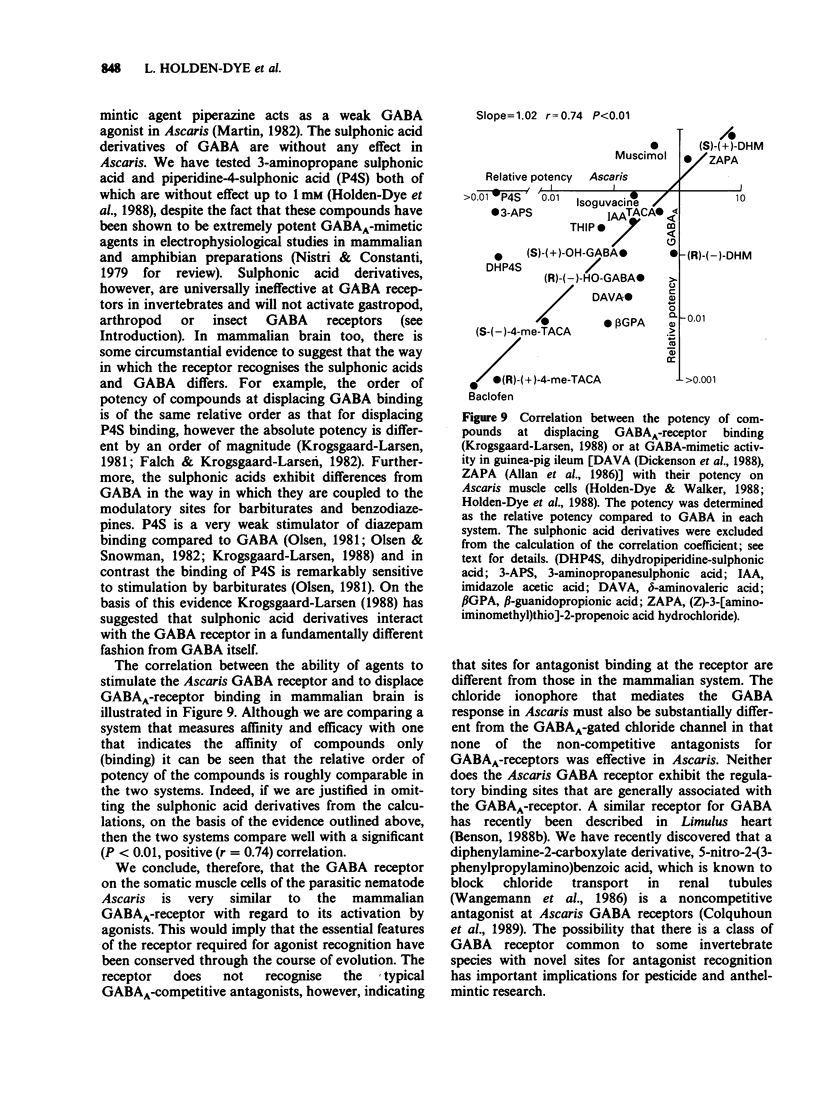
- BOISTEL J., FATT P. Membrane permeability change during inhibitory transmitter action in crustacean muscle. J Physiol. 1958 Nov 10;144(1):176–191. doi: 10.1113/jphysiol.1958.sp006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokisch A. J., Walker R. J. The ionic mechanism associated with the action of putative transmitters on identified neurons of the snail, Helix aspersa. Comp Biochem Physiol C. 1986;84(2):231–241. doi: 10.1016/0742-8413(86)90088-5. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., WATKINS J. C. Analogues of glutamic and gamma-amino-n-butyric acids having potent actions on mammalian neurones. Nature. 1961 Sep 2;191:1010–1011. doi: 10.1038/1911010a0. [DOI] [PubMed] [Google Scholar]
- Campochiaro P., Schwarcz R., Coyle J. T. GABA receptor binding in rat striatum: localization and effects of denervation. Brain Res. 1977 Nov 18;136(3):501–511. doi: 10.1016/0006-8993(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Constanti A. The "mixed" effect of picrotoxin on the GABA dose/conductance relation recorded from lobster muscle. Neuropharmacology. 1978 Mar;17(3):159–167. doi: 10.1016/0028-3908(78)90095-3. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A. Amino acid transmitters in the mammalian central nervous system. Ergeb Physiol. 1974;69(0):97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- Dickenson H. W., Allan R. D., Ong J., Johnston G. A. GABAB receptor antagonist and GABAA receptor agonist properties of a delta-aminovaleric acid derivative, Z-5-aminopent-2-enoic acid. Neurosci Lett. 1988 Apr 12;86(3):351–355. doi: 10.1016/0304-3940(88)90509-5. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. The effect of inhibitory nerve impulses on a crustacean muscle fibre. J Physiol. 1953 Aug;121(2):374–389. doi: 10.1113/jphysiol.1953.sp004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falch E., Krogsgaard-Larsen P. The binding of the GABA agonist [3H]THIP to rat brain synaptic membranes. J Neurochem. 1982 Apr;38(4):1123–1129. doi: 10.1111/j.1471-4159.1982.tb05357.x. [DOI] [PubMed] [Google Scholar]
- Gant D. B., Eldefrawi M. E., Eldefrawi A. T. Cyclodiene insecticides inhibit GABAA receptor-regulated chloride transport. Toxicol Appl Pharmacol. 1987 May;88(3):313–321. doi: 10.1016/0041-008x(87)90206-7. [DOI] [PubMed] [Google Scholar]
- Holden-Dye L., Walker R. J. ZAPA, (Z)-3-[(aminoiminomethyl)thio]-2-propenoic acid hydrochloride, a potent agonist at GABA-receptors on the Ascaris muscle cell. Br J Pharmacol. 1988 Sep;95(1):3–5. doi: 10.1111/j.1476-5381.1988.tb16540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori N., Ikeda K., Roberts E. Muscimol, GABA and picrotoxin: effects on membrane conductance of a crustacean neuron. Brain Res. 1978 Feb 10;141(2):364–370. doi: 10.1016/0006-8993(78)90207-x. [DOI] [PubMed] [Google Scholar]
- Johnson C. D., Stretton A. O. GABA-immunoreactivity in inhibitory motor neurons of the nematode Ascaris. J Neurosci. 1987 Jan;7(1):223–235. doi: 10.1523/JNEUROSCI.07-01-00223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P. GABA synaptic mechanisms: stereochemical and conformational requirements. Med Res Rev. 1988 Jan-Mar;8(1):27–56. doi: 10.1002/med.2610080103. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P., Nielsen L., Falch E., Curtis D. R. GABA agonists. Resolution, absolute stereochemistry, and enantioselectivity of (S)-(+)- and (R)-(-)-dihydromuscimol. J Med Chem. 1985 Nov;28(11):1612–1617. doi: 10.1021/jm00149a012. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P. gamma-Aminobutyric acid agonists, antagonists, and uptake inhibitors. Design and therapeutic aspects. J Med Chem. 1981 Dec;24(12):1377–1383. doi: 10.1021/jm00144a001. [DOI] [PubMed] [Google Scholar]
- Lees G., Beadle D. J., Neumann R., Benson J. A. Responses to GABA by isolated insect neuronal somata: pharmacology and modulation by a benzodiazepine and a barbiturate. Brain Res. 1987 Jan 20;401(2):267–278. doi: 10.1016/0006-8993(87)91411-9. [DOI] [PubMed] [Google Scholar]
- Martin R. J. Electrophysiological effects of piperazine and diethylcarbamazine on Ascaris suum somatic muscle. Br J Pharmacol. 1982 Oct;77(2):255–265. doi: 10.1111/j.1476-5381.1982.tb09294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. J. The effect of gamma-aminobutyric acid on the input conductance and membrane potential of Ascaris muscle. Br J Pharmacol. 1980;71(1):99–106. doi: 10.1111/j.1476-5381.1980.tb10914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokizawa F., Reuben J. P., Grundfest H. Ionic permeability of the inhibitory postsynaptic membrane of lobster muscle fibers. J Gen Physiol. 1969 Oct;54(4):437–461. doi: 10.1085/jgp.54.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistri A., Constanti A. Pharmacological characterization of different types of GABA and glutamate receptors in vertebrates and invertebrates. Prog Neurobiol. 1979;13(2):117–235. doi: 10.1016/0301-0082(79)90016-9. [DOI] [PubMed] [Google Scholar]
- Olsen R. W. GABA-benzodiazepine-barbiturate receptor interactions. J Neurochem. 1981 Jul;37(1):1–13. doi: 10.1111/j.1471-4159.1981.tb05284.x. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Snowman A. M. Chloride-dependent enhancement by barbiturates of gamma-aminobutyric acid receptor binding. J Neurosci. 1982 Dec;2(12):1812–1823. doi: 10.1523/JNEUROSCI.02-12-01812.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott S. M., Kerkut G. A., Walker R. J. The actions of picrotoxin, strychnine, bicuculline and other convulsants and antagonists on the responses to acetylcholine glutamic acid and gamma-aminobutyric acid on Helix neurones. Comp Biochem Physiol C. 1977;57(2):107–116. doi: 10.1016/0306-4492(77)90054-5. [DOI] [PubMed] [Google Scholar]
- Pinnock R. D., David J. A., Sattelle D. B. Ionic events following GABA receptor activation in an identified insect motor neuron. Proc R Soc Lond B Biol Sci. 1988 Jan 22;232(1269):457–470. doi: 10.1098/rspb.1988.0007. [DOI] [PubMed] [Google Scholar]
- Pitman R. M., Kerkut G. A. Comparison of the actions of iontophoretically applied acetylcholine and gamma aminobutyric acid with the EPSP and IPSP in cockroach central neurons. Comp Gen Pharmacol. 1970 Jun;1(2):221–230. doi: 10.1016/0010-4035(70)90056-x. [DOI] [PubMed] [Google Scholar]
- Robinson T., MacAllan D., Lunt G., Battersby M. gamma-Aminobutyric acid receptor complex of insect CNS: characterization of a benzodiazepine binding site. J Neurochem. 1986 Dec;47(6):1955–1962. doi: 10.1111/j.1471-4159.1986.tb13114.x. [DOI] [PubMed] [Google Scholar]
- Sattelle D. B., Pinnock R. D., Wafford K. A., David J. A. GABA receptors on the cell-body membrane of an identified insect motor neuron. Proc R Soc Lond B Biol Sci. 1988 Jan 22;232(1269):443–456. doi: 10.1098/rspb.1988.0006. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Duce I. R. Pharmacology of GABA receptors on skeletal muscle fibres of the locust (Schistocerca gregaria). Comp Biochem Physiol C. 1987;86(2):305–311. doi: 10.1016/0742-8413(87)90084-3. [DOI] [PubMed] [Google Scholar]
- Slater N. T., Carpenter D. O. A study of the cholinolytic actions of strychnine using the technique of concentration jump relaxation analysis. Cell Mol Neurobiol. 1984 Sep;4(3):263–271. doi: 10.1007/BF00733589. [DOI] [PubMed] [Google Scholar]
- Squires R. F., Casida J. E., Richardson M., Saederup E. [35S]t-butylbicyclophosphorothionate binds with high affinity to brain-specific sites coupled to gamma-aminobutyric acid-A and ion recognition sites. Mol Pharmacol. 1983 Mar;23(2):326–336. [PubMed] [Google Scholar]
- Steward E. G., Borthwick P. W., Clarke G. R., Warner D. Agonism and antagonism of gamma-aminobutyric acid. Nature. 1975 Aug 14;256(5518):600–602. doi: 10.1038/256600a0. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. Anion permeability of the inhibitory post-synaptic membrane of the crayfish neuromuscular junction. J Physiol. 1967 Aug;191(3):575–590. doi: 10.1113/jphysiol.1967.sp008269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop B., Christensen T. A., Hildebrand J. G. GABA-mediated synaptic inhibition of projection neurons in the antennal lobes of the sphinx moth, Manduca sexta. J Comp Physiol A. 1987 Jun;161(1):23–32. doi: 10.1007/BF00609452. [DOI] [PubMed] [Google Scholar]
- Walker R. J., Crossman A. R., Woodruff G. N., Kerkut G. A. The effect of bicuculline on the gamma-aminobutyric acid (GABA) receptors of neurons of Periplaneta americana and Helix aspersa. Brain Res. 1971 Oct 8;33(1):75–82. doi: 10.1016/0006-8993(71)90306-4. [DOI] [PubMed] [Google Scholar]
- Walker R. J., Holden-Dye L. Commentary on the evolution of transmitters, receptors and ion channels in invertebrates. Comp Biochem Physiol A Comp Physiol. 1989;93(1):25–39. doi: 10.1016/0300-9629(89)90188-6. [DOI] [PubMed] [Google Scholar]
- Walker R. J., Roberts C. J. The pharmacology of Limulus central neurons. Comp Biochem Physiol C. 1982;72(2):391–401. doi: 10.1016/0306-4492(82)90110-1. [DOI] [PubMed] [Google Scholar]
- Wangemann P., Wittner M., Di Stefano A., Englert H. C., Lang H. J., Schlatter E., Greger R. Cl(-)-channel blockers in the thick ascending limb of the loop of Henle. Structure activity relationship. Pflugers Arch. 1986;407 (Suppl 2):S128–S141. doi: 10.1007/BF00584942. [DOI] [PubMed] [Google Scholar]
- Wann K. T. The electrophysiology of the somatic muscle cells of Ascaris suum and Ascaridia galli. Parasitology. 1987 Jun;94(Pt 3):555–566. doi: 10.1017/s003118200005589x. [DOI] [PubMed] [Google Scholar]
- Yarowsky P. J., Carpenter D. O. Receptors for gamma-aminobutyric acid (GABA) on Aplysia neurons. Brain Res. 1978 Apr 7;144(1):75–94. doi: 10.1016/0006-8993(78)90436-5. [DOI] [PubMed] [Google Scholar]


