Abstract
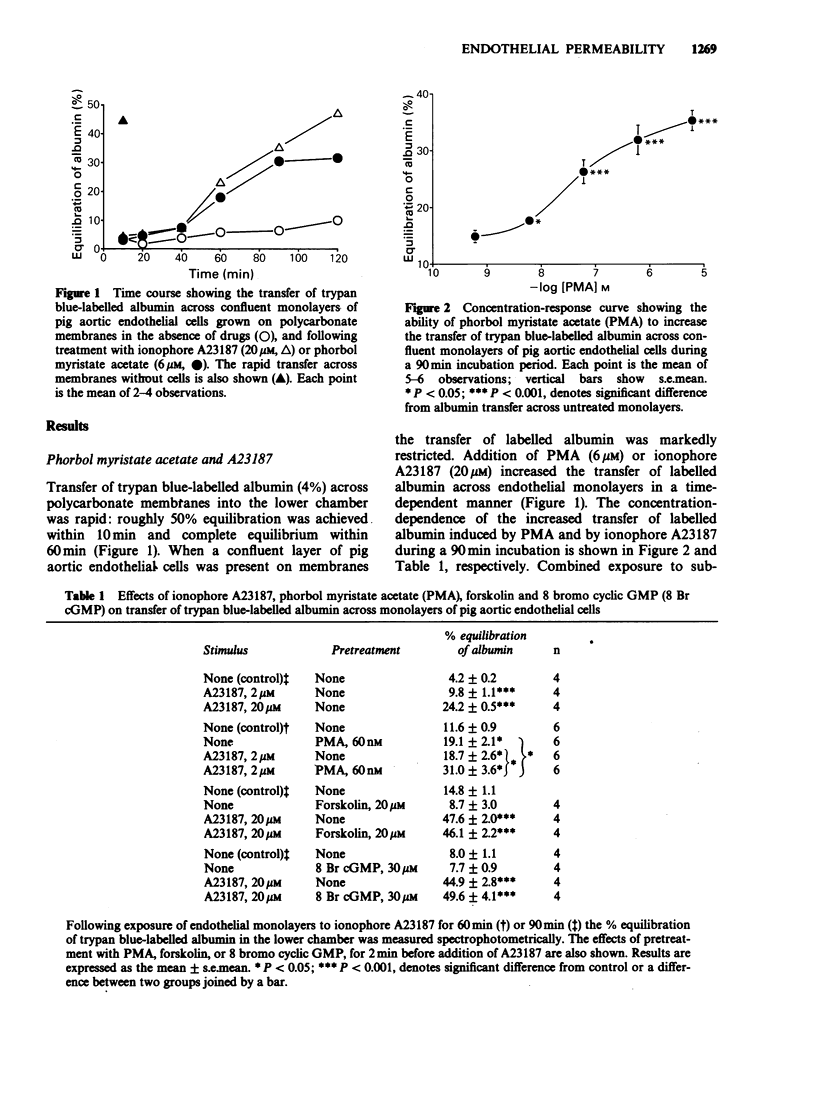
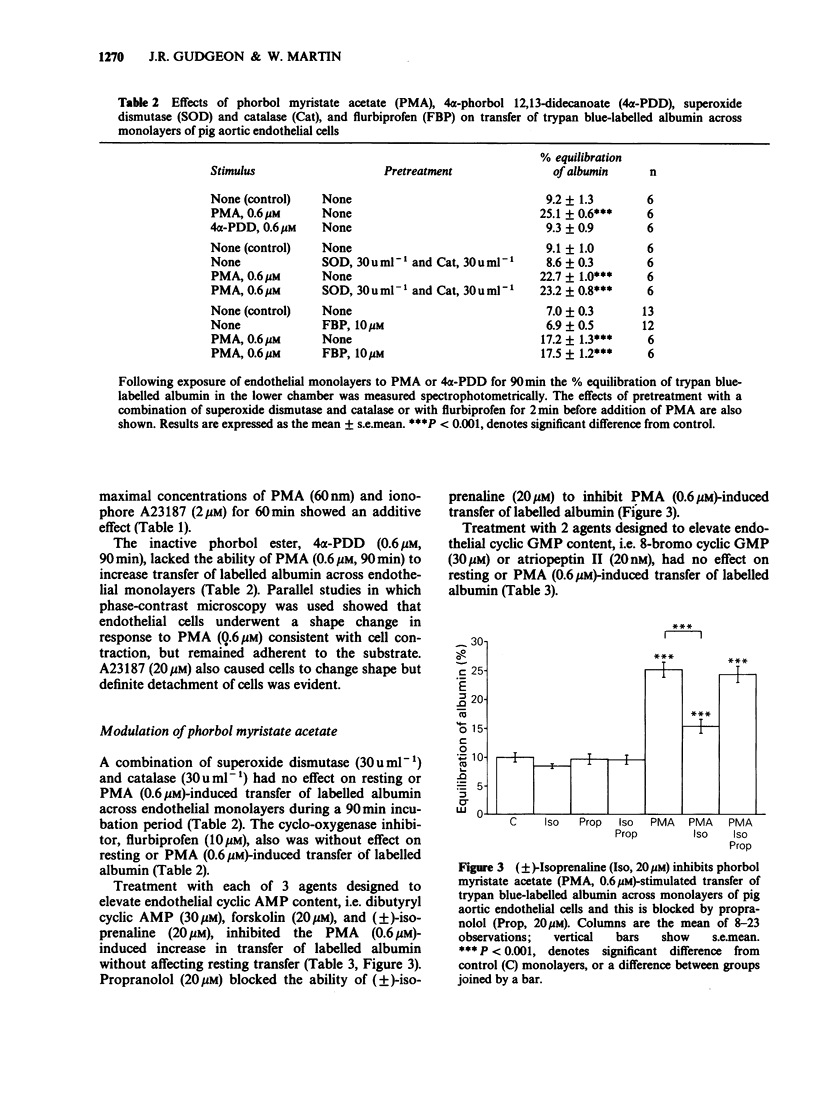
1. An in vitro model of the arterial endothelial barrier was established in which transfer of trypan blue-labelled albumin across confluent monolayers of pig aortic endothelial cells grown on polycarbonate membranes was measured. 2. A range of inflammatory mediators, i.e. histamine, bradykinin, platelet activating factor and thrombin, had no effect on the transfer of labelled albumin across aortic endothelial monolayers. 3. Calcium ionophore A23187 and the phorbol ester, phorbol myristate acetate (PMA), each induced concentration-dependent increases in transfer of labelled albumin. These increases were associated with changes in cell shape, consistent with endothelial contraction. Ionophore A23187 caused some detachment of cells. 4. The ability of PMA to increase transfer of labelled albumin probably results from activation of protein kinase C since it was not shared by the inactive analogue, 4 alpha-phorbol 12,13-didecanoate. 5. Neither a combination of superoxide dismutase and catalase nor the cyclo-oxygenase inhibitor, flurbiprofen, affected resting or PMA-induced increases in albumin transfer. Oxygen-derived free radicals and prostaglandins appear not to be involved in the response to PMA. 6. Each of three procedures designed to elevate adenosine 3':5'-cyclic monophosphate (cyclic AMP) levels, i.e. dibutyryl cyclic AMP, forskolin and (+/-)-isoprenaline, reduced the ability of PMA to promote increased transfer of labelled albumin but had no effect on resting transfer. The effect of (+/-)-isoprenaline was abolished by the beta-adrenoceptor blocking agent, propranolol.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker C. G., Nachman R. L. Contractile proteins of endothelial cells, platelets and smooth muscle. Am J Pathol. 1973 Apr;71(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- Chander C. L., Moore A. R., Desa F. M., Howat D., Willoughby D. A. The local modulation of vascular permeability by endothelial cell derived products. J Pharm Pharmacol. 1988 Oct;40(10):745–746. doi: 10.1111/j.2042-7158.1988.tb07011.x. [DOI] [PubMed] [Google Scholar]
- Del Vecchio P. J., Siflinger-Birnboim A., Shepard J. M., Bizios R., Cooper J. A., Malik A. B. Endothelial monolayer permeability to macromolecules. Fed Proc. 1987 Jun;46(8):2511–2515. [PubMed] [Google Scholar]
- Duffey M. E., Hainau B., Ho S., Bentzel C. J. Regulation of epithelial tight junction permeability by cyclic AMP. Nature. 1981 Dec 3;294(5840):451–453. doi: 10.1038/294451a0. [DOI] [PubMed] [Google Scholar]
- Federation of American Societies for Experimental Biology. 72nd annual meeting. Las Vegas, Nevada, May 1-5, 1988. Abstracts of papers 1-3785. FASEB J. 1988 Mar 15;2(4):A297–A946. [PubMed] [Google Scholar]
- Federation of American Societies for Experimental Biology. 72nd annual meeting. Las Vegas, Nevada, May 1-5, 1988. Abstracts of papers 1-3785. FASEB J. 1988 Mar 15;2(4):A297–A946. [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Inhibition of collagen gelation by action of the superoxide radical. Arthritis Rheum. 1979 Mar;22(3):251–259. doi: 10.1002/art.1780220307. [DOI] [PubMed] [Google Scholar]
- Grigorian G. Y., Ryan U. S. Platelet-activating factor effects on bovine pulmonary artery endothelial cells. Circ Res. 1987 Sep;61(3):389–395. doi: 10.1161/01.res.61.3.389. [DOI] [PubMed] [Google Scholar]
- Hennig B., Chow C. K. Lipid peroxidation and endothelial cell injury: implications in atherosclerosis. Free Radic Biol Med. 1988;4(2):99–106. doi: 10.1016/0891-5849(88)90070-6. [DOI] [PubMed] [Google Scholar]
- Killackey J. J., Johnston M. G., Movat H. Z. Increased permeability of microcarrier-cultured endothelial monolayers in response to histamine and thrombin. A model for the in vitro study of increased vasopermeability. Am J Pathol. 1986 Jan;122(1):50–61. [PMC free article] [PubMed] [Google Scholar]
- Litchfield D. W., Ball E. H. Phosphorylation of the cytoskeletal protein talin by protein kinase C. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1276–1283. doi: 10.1016/0006-291x(86)90388-8. [DOI] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G., Gilmore V., Leventhal M. On the mechanism of vascular leakage caused by histaminetype mediators. A microscopic study in vivo. Circ Res. 1967 Dec;21(6):833–847. doi: 10.1161/01.res.21.6.833. [DOI] [PubMed] [Google Scholar]
- Marciniak D. L., Dobbins D. E., Maciejko J. J., Scott J. B., Haddy F. J., Grega G. J. Antagonism of histamine edema formation by catecholamines. Am J Physiol. 1978 Feb;234(2):H180–H185. doi: 10.1152/ajpheart.1978.234.2.H180. [DOI] [PubMed] [Google Scholar]
- Martin W., White D. G., Henderson A. H. Endothelium-derived relaxing factor and atriopeptin II elevate cyclic GMP levels in pig aortic endothelial cells. Br J Pharmacol. 1988 Jan;93(1):229–239. doi: 10.1111/j.1476-5381.1988.tb11426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Superoxide anion release by human endothelial cells: synergism between a phorbol ester and a calcium ionophore. J Cell Physiol. 1986 May;127(2):207–210. doi: 10.1002/jcp.1041270203. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Pearson J. D., Carleton J. S., Hutchings A. Prostacyclin release stimulated by thrombin or bradykinin in porcine endothelial cells cultured from aorta and umbilical vein. Thromb Res. 1983 Jan 15;29(2):115–124. doi: 10.1016/0049-3848(83)90133-0. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Freeman B. A. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Harker L. Hyperlipidemia and atherosclerosis. Science. 1976 Sep 17;193(4258):1094–1100. doi: 10.1126/science.822515. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Gallin J. I. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J Cell Biol. 1986 Dec;103(6 Pt 1):2379–2387. doi: 10.1083/jcb.103.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shasby D. M., Lind S. E., Shasby S. S., Goldsmith J. C., Hunninghake G. W. Reversible oxidant-induced increases in albumin transfer across cultured endothelium: alterations in cell shape and calcium homeostasis. Blood. 1985 Mar;65(3):605–614. [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Sullivan J. M., Peach M. J. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982 Nov;51(5):657–661. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- Svensjö E., Arfors K. E., Raymond R. M., Grega G. J. Morphological and physiological correlation of bradykinin-induced macromolecular efflux. Am J Physiol. 1979 Apr;236(4):H600–H606. doi: 10.1152/ajpheart.1979.236.4.H600. [DOI] [PubMed] [Google Scholar]
- Svensjö E., Grega G. J. Evidence for endothelial cell-mediated regulation of macromolecular permeability by postcapillary venules. Fed Proc. 1986 Feb;45(2):89–95. [PubMed] [Google Scholar]
- Werth D. K., Niedel J. E., Pastan I. Vinculin, a cytoskeletal substrate of protein kinase C. J Biol Chem. 1983 Oct 10;258(19):11423–11426. [PubMed] [Google Scholar]
- Williams T. J. Interactions between prostaglandins, leukotrienes and other mediators of inflammation. Br Med Bull. 1983 Jul;39(3):239–242. doi: 10.1093/oxfordjournals.bmb.a071826. [DOI] [PubMed] [Google Scholar]


