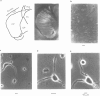
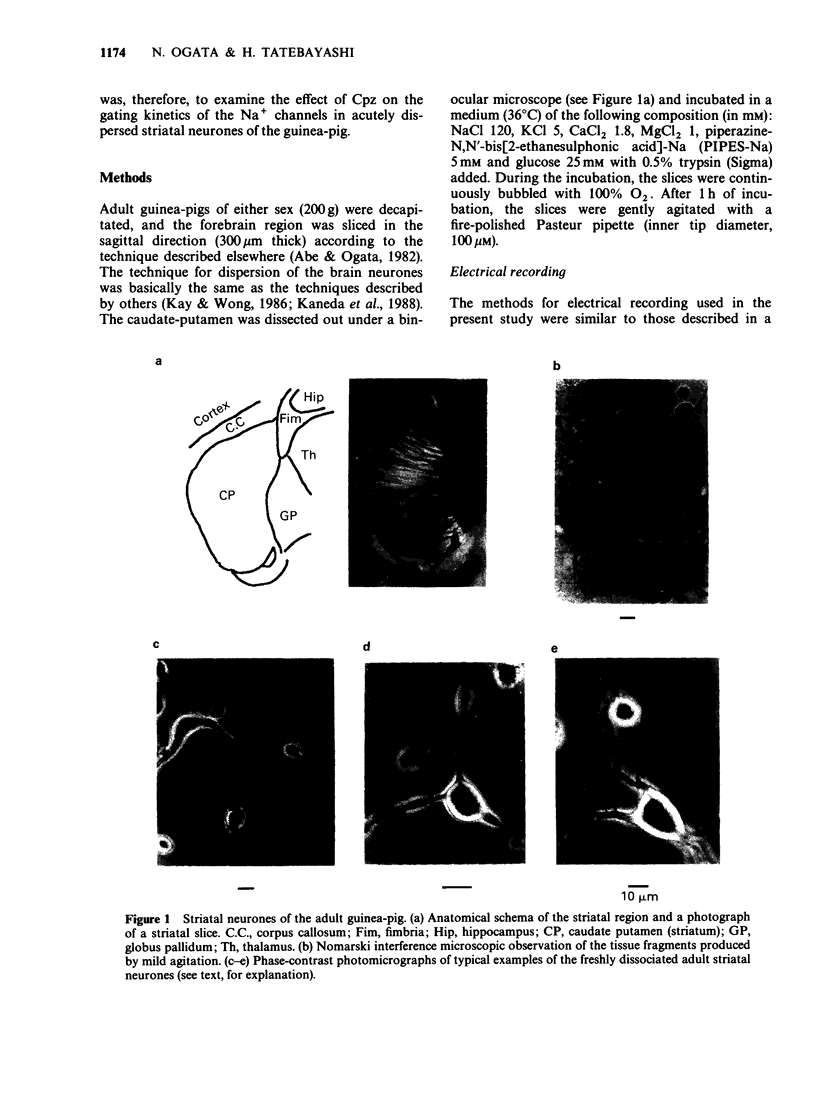
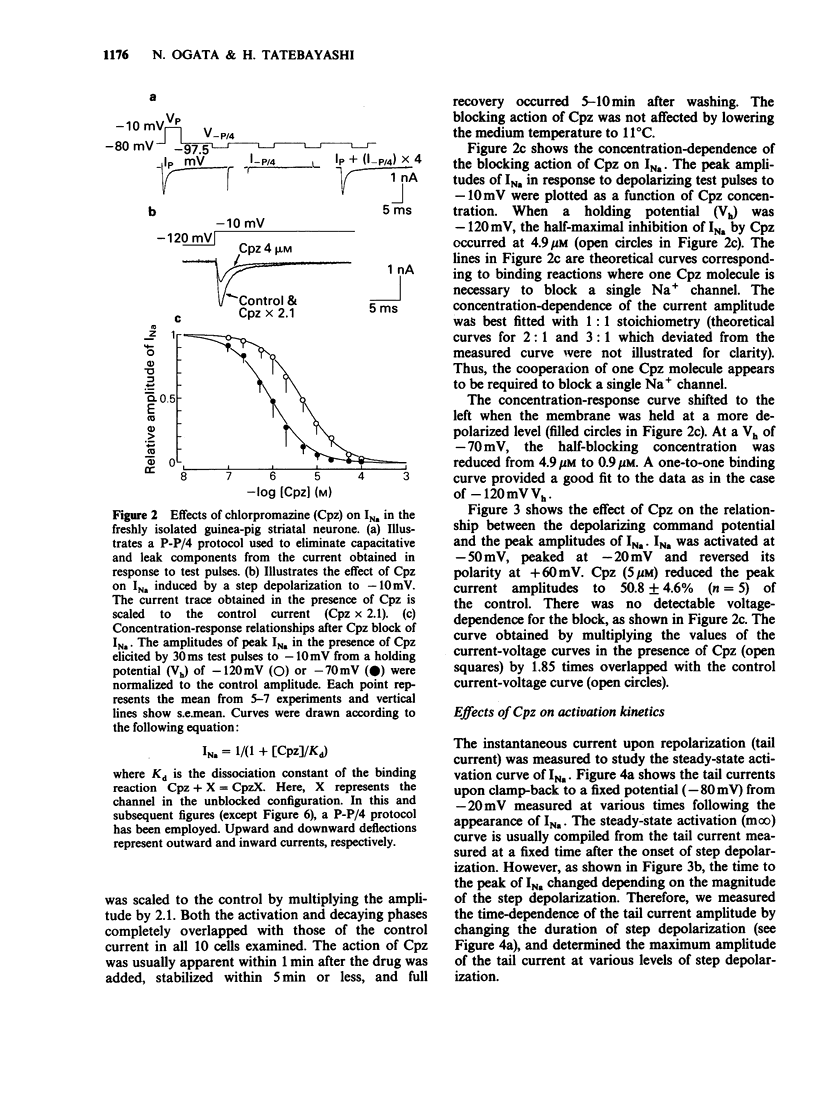
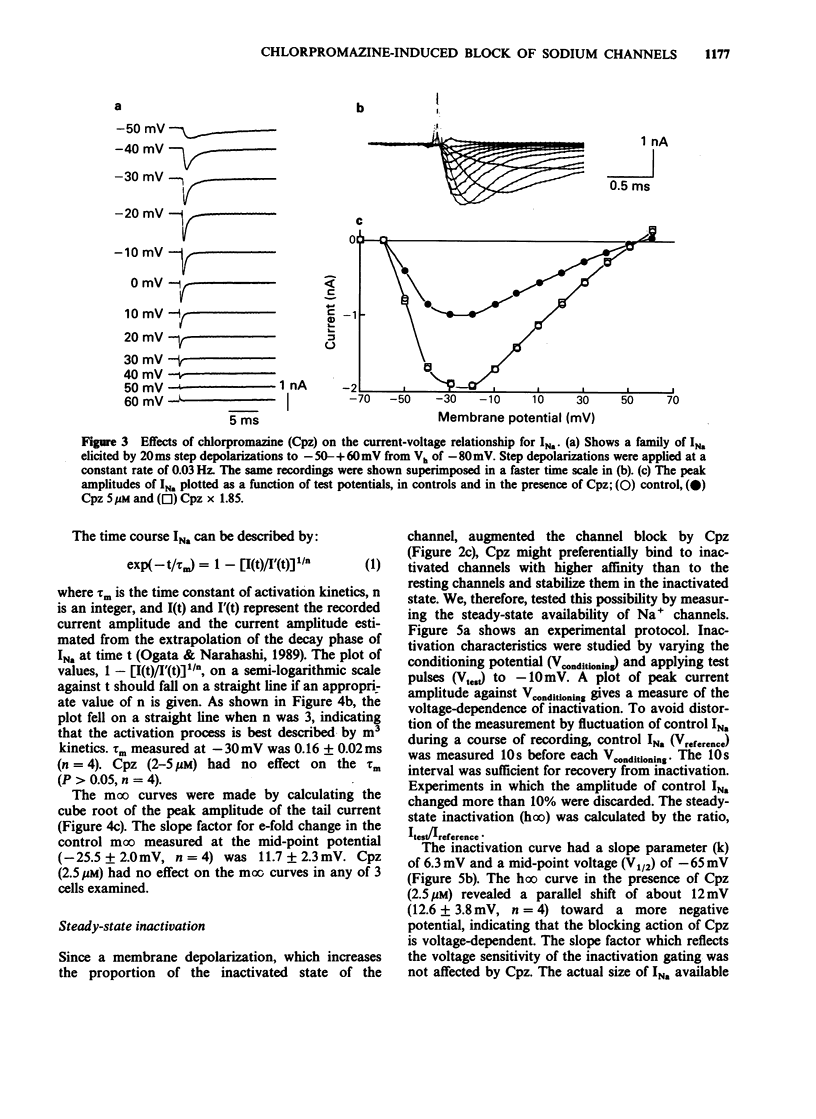
Abstract
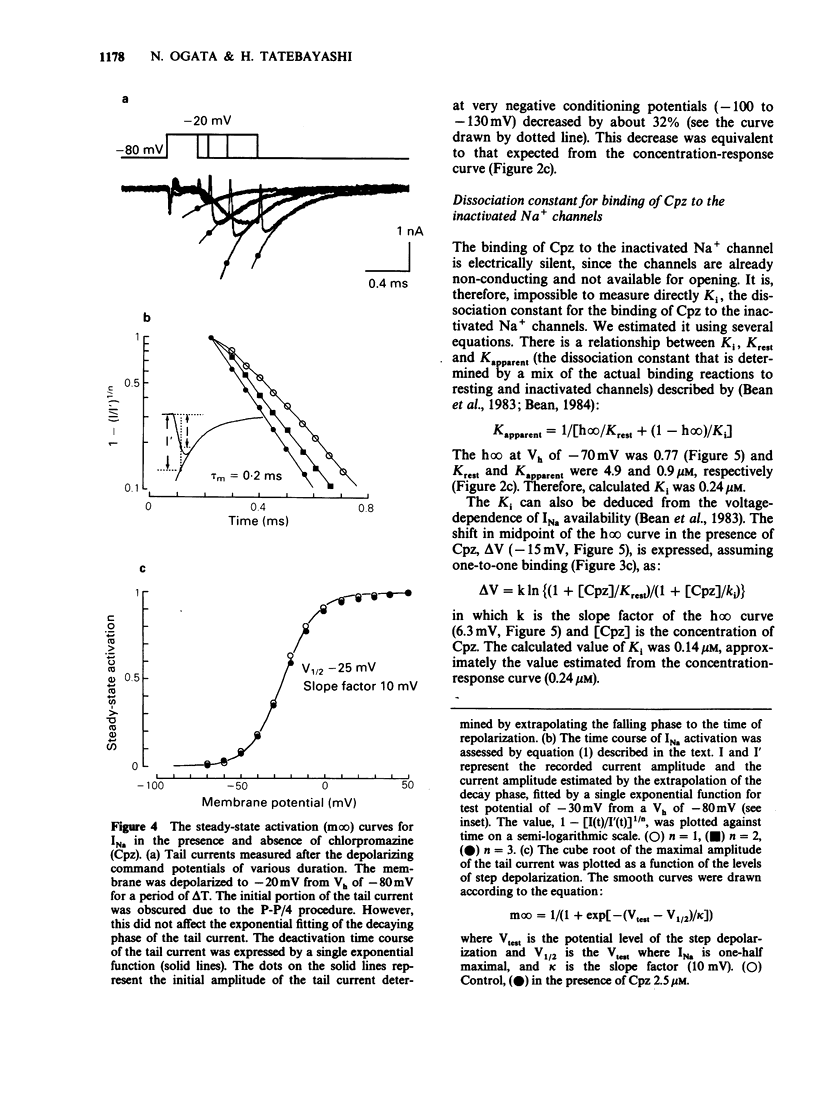
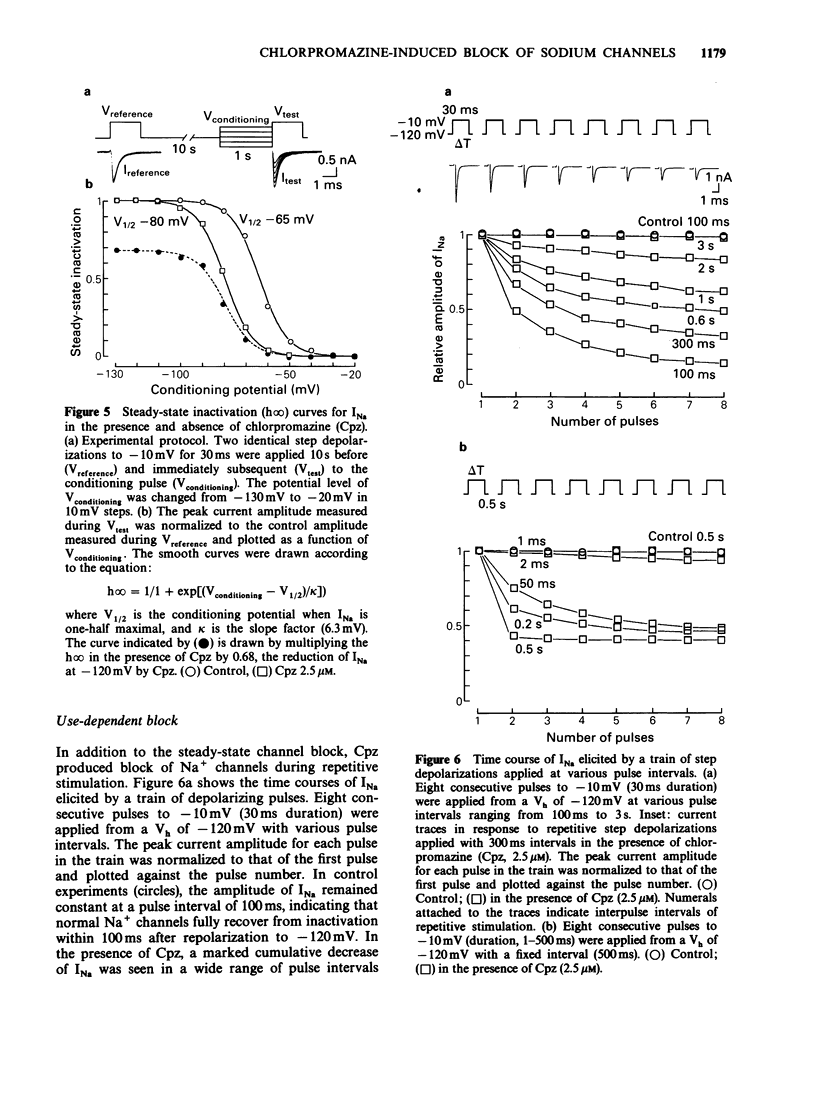
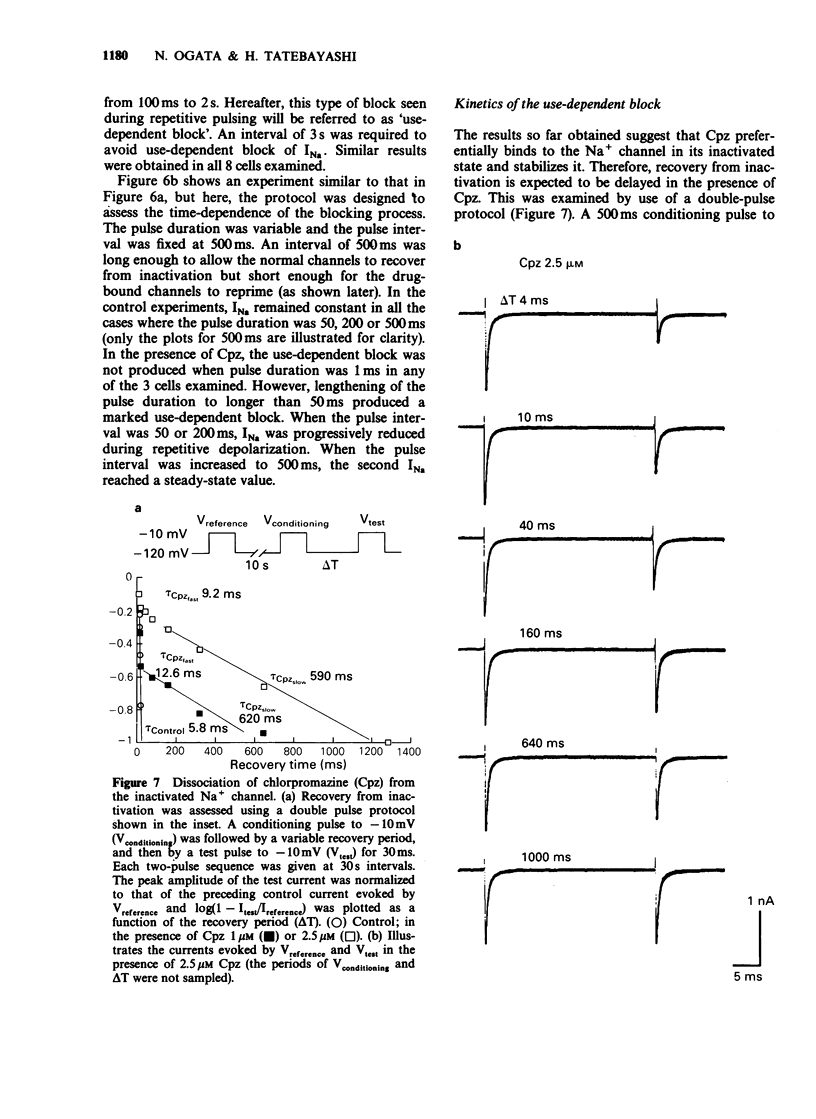
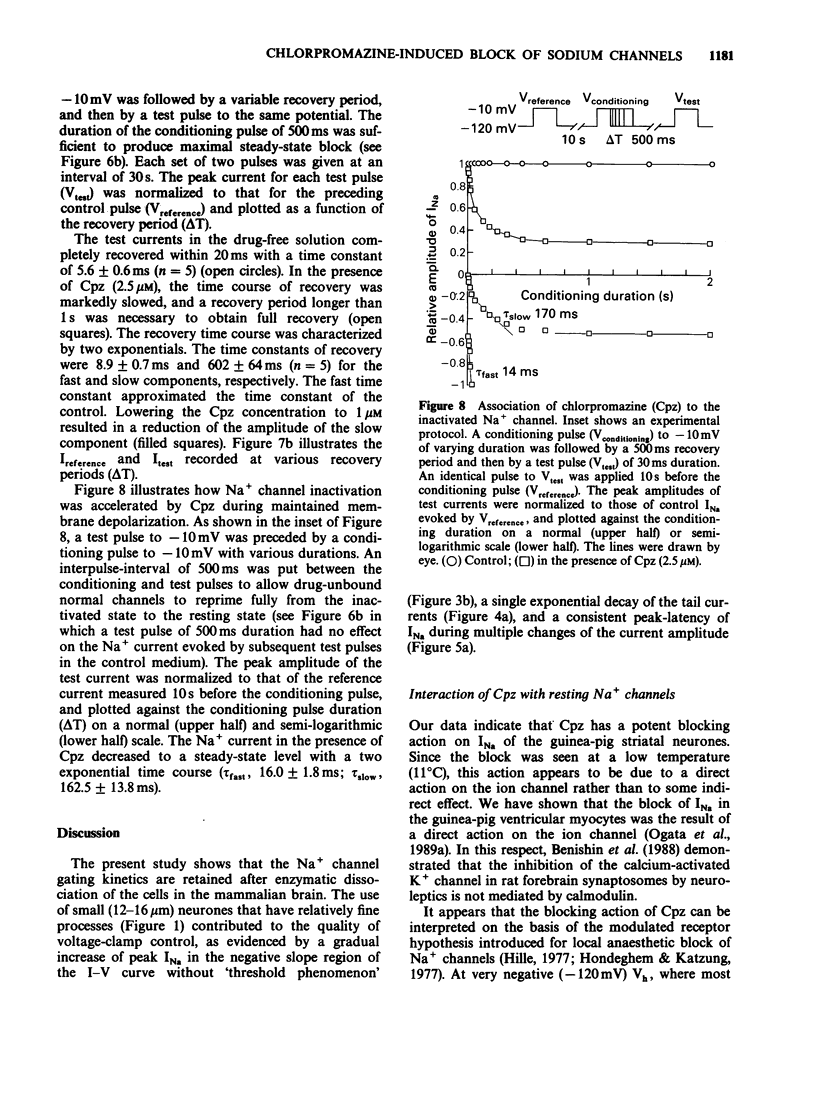
1. The neurones of the striatum were freshly dissociated from the adult guinea-pig brain by enzymatic and mechanical treatments. Sodium channel current kinetics in these neurones were measured using a whole cell variation of the patch-clamp technique. 2. Chlorpromazine, a neuroleptic, in micromolar concentrations reversibly reduced the amplitude of the sodium currents. Activation and inactivation time constants were not affected. The inhibition followed one-to-one binding stoichiometry. 3. The concentration-response curve shifted to the left when the holding potential was less negative. The EC50 shifted from 4.8 microM to 0.9 microM when the holding potential was changed from -120 mV to -70 mV. 4. The steady-state activation curve of the sodium current was not affected by chlorpromazine, whereas the steady-state inactivation curve was shifted in the negative direction. Consequently, the window current which is normally present at a potential range around -50 mV was decreased in the presence of chlorpromazine. 5. Successive sodium currents evoked by a train of depolarizing pulses (30 ms duration) to -10 mV showed a cumulative decrease in size during the application of chlorpromazine. However, such 'use-dependent' block was not observed when the pulse duration was reduced to 1 ms. 6. The recovery from inactivation in the presence of chlorpromazine, was expressed as a second order process. The faster component was similar to the recovery time course of the normal sodium channels. The slower component accounted for the use-dependent effect of chlorpromazine. 7. The results indicate that chlorpromazine binds to the resting sodium channels producing steady-state block at a very negative holding potential. When the membrane is depolarized, chlorpromazine binds to the inactivated form of the sodium channels with much higher affinity and stabilizes them in the inactivated state, slowing their kinetics.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe H., Ogata N. Ionic mechanism for the osmotically-induced depolarization in neurones of the guinea-pig supraoptic nucleus in vitro. J Physiol. 1982 Jun;327:157–171. doi: 10.1113/jphysiol.1982.sp014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Cohen C. J., Tsien R. W. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983 May;81(5):613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benishin C. G., Krueger B. K., Blaustein M. P. Phenothiazines and haloperidol block Ca-activated K channels in rat forebrain synaptosomes. Mol Pharmacol. 1988 Feb;33(2):195–201. [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. T., Wilson C. J., Kitai S. T. A Golgi study of rat neostriatal neurons: light microscopic analysis. J Comp Neurol. 1982 Jun 20;208(2):107–126. doi: 10.1002/cne.902080202. [DOI] [PubMed] [Google Scholar]
- Cooper T. B., Simpson G. M., Lee J. H. Thymoleptic and neuroleptic drug plasma levels in psychiatry: current status. Int Rev Neurobiol. 1976;19:269–309. doi: 10.1016/s0074-7742(08)60706-0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977 Nov 14;472(3-4):373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Huguenard J. R., Alger B. E. Whole-cell voltage-clamp study of the fading of GABA-activated currents in acutely dissociated hippocampal neurons. J Neurophysiol. 1986 Jul;56(1):1–18. doi: 10.1152/jn.1986.56.1.1. [DOI] [PubMed] [Google Scholar]
- Kaneda M., Nakamura H., Akaike N. Mechanical and enzymatic isolation of mammalian CNS neurons. Neurosci Res. 1988 Apr;5(4):299–315. doi: 10.1016/0168-0102(88)90032-6. [DOI] [PubMed] [Google Scholar]
- Kay A. R., Wong R. K. Isolation of neurons suitable for patch-clamping from adult mammalian central nervous systems. J Neurosci Methods. 1986 May;16(3):227–238. doi: 10.1016/0165-0270(86)90040-3. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Lu E. J., Brown W. J. The developing caudate nucleus in the euthyroid and hypothyroid rat. J Comp Neurol. 1977 Jan 15;171(2):261–284. doi: 10.1002/cne.901710209. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Tsunoo A., Yoshii M. Characterization of two types of calcium channels in mouse neuroblastoma cells. J Physiol. 1987 Feb;383:231–249. doi: 10.1113/jphysiol.1987.sp016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N., Narahashi T. Block of sodium channels by psychotropic drugs in single guinea-pig cardiac myocytes. Br J Pharmacol. 1989 Jul;97(3):905–913. doi: 10.1111/j.1476-5381.1989.tb12031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N., Nishimura M., Narahashi T. Kinetics of chlorpromazine block of sodium channels in single guinea pig cardiac myocytes. J Pharmacol Exp Ther. 1989 Feb;248(2):605–613. [PubMed] [Google Scholar]
- Ogata N., Yoshii M., Narahashi T. Psychotropic drugs block voltage-gated ion channels in neuroblastoma cells. Brain Res. 1989 Jan 2;476(1):140–144. doi: 10.1016/0006-8993(89)91546-1. [DOI] [PubMed] [Google Scholar]