Abstract
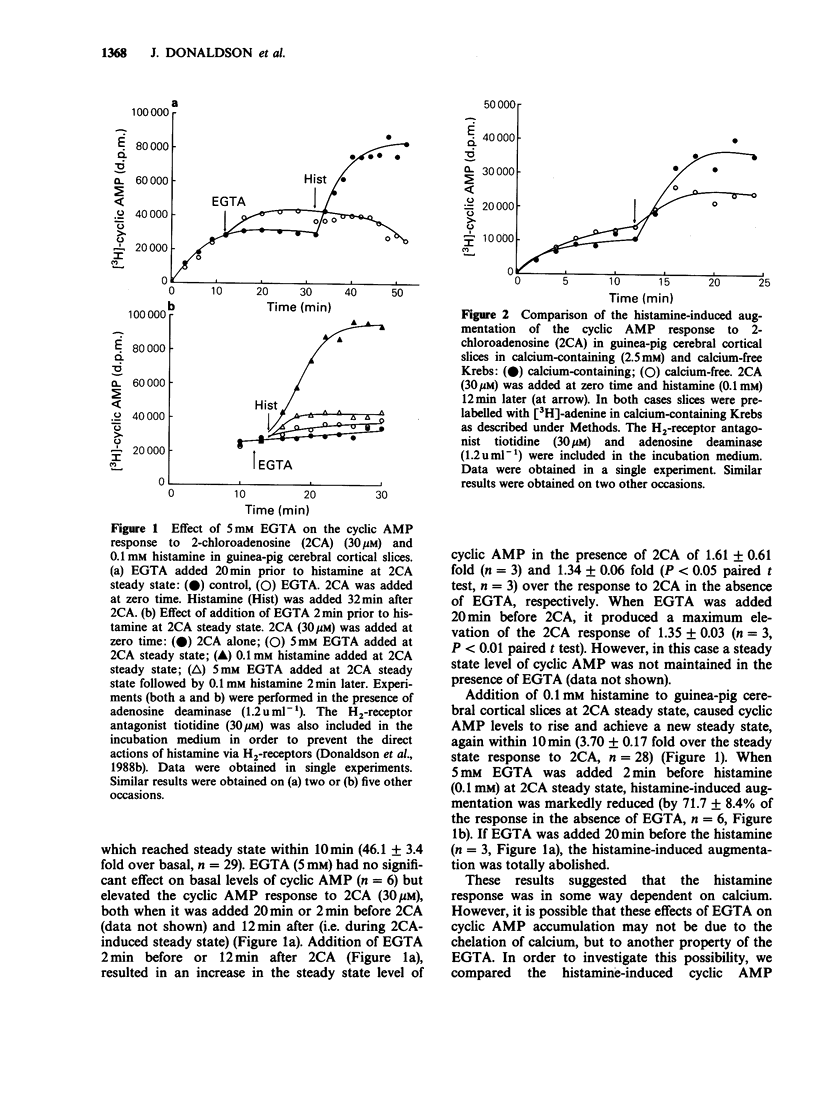
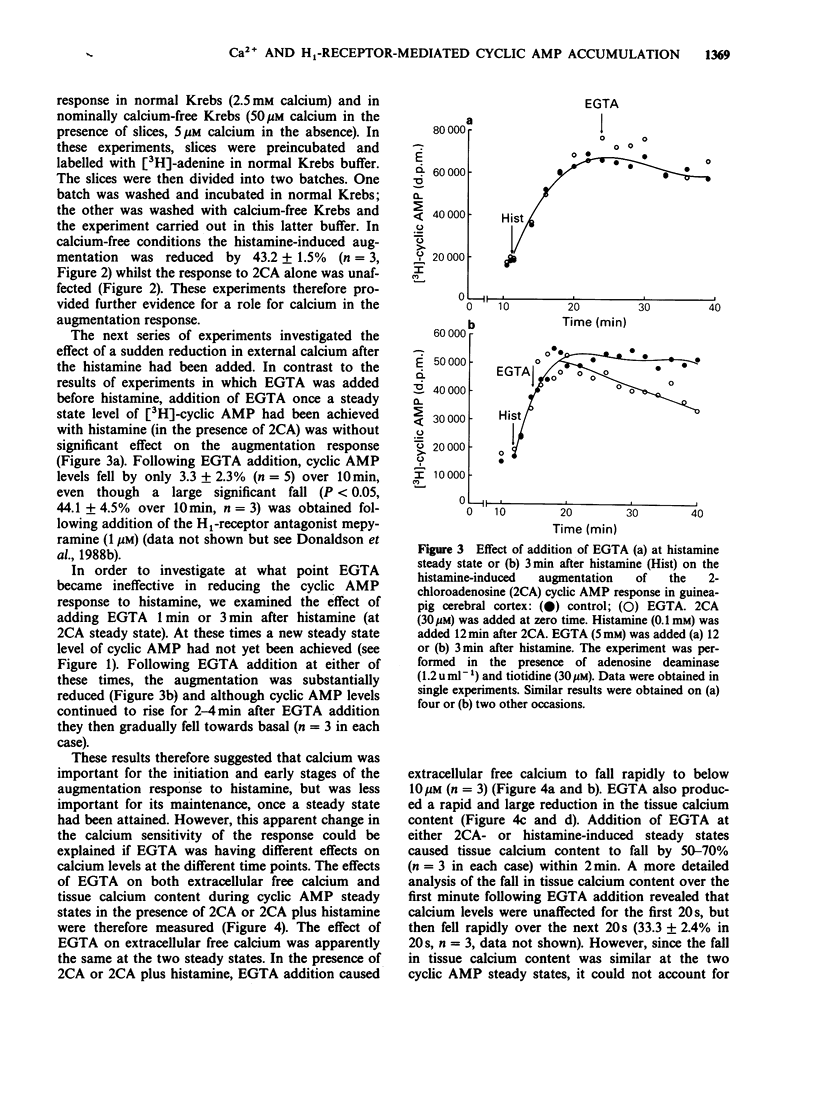
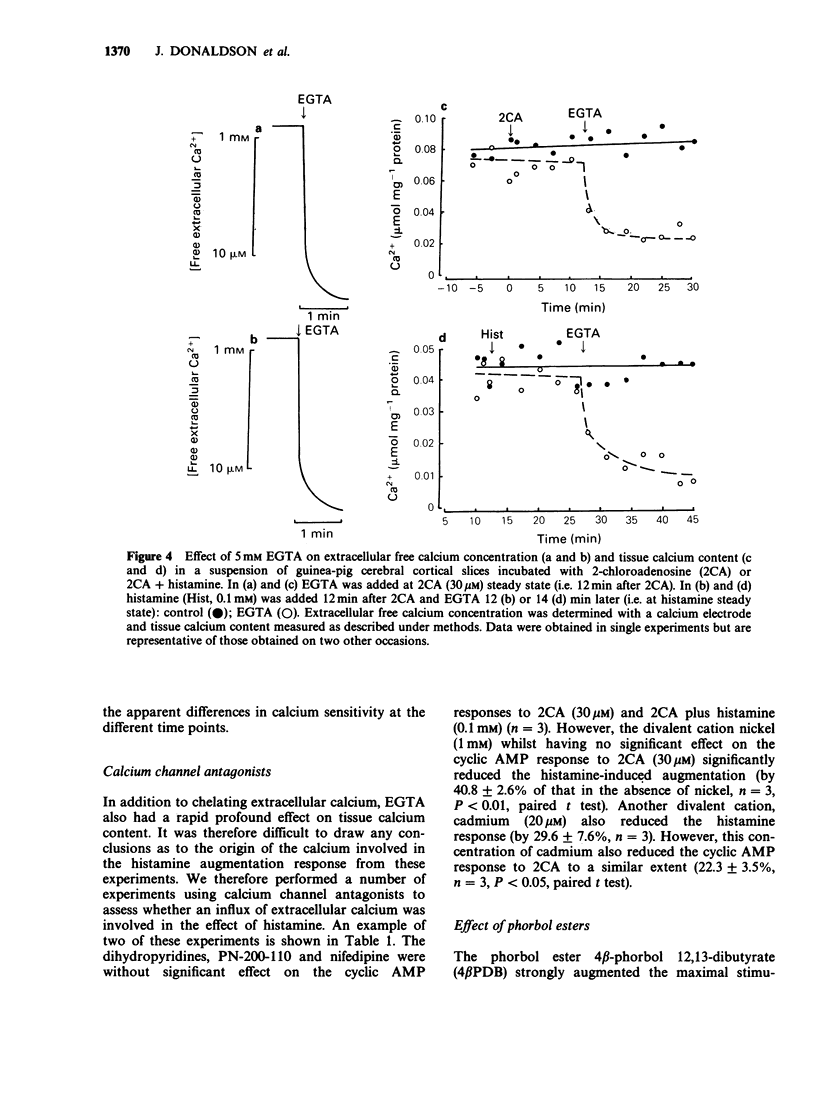
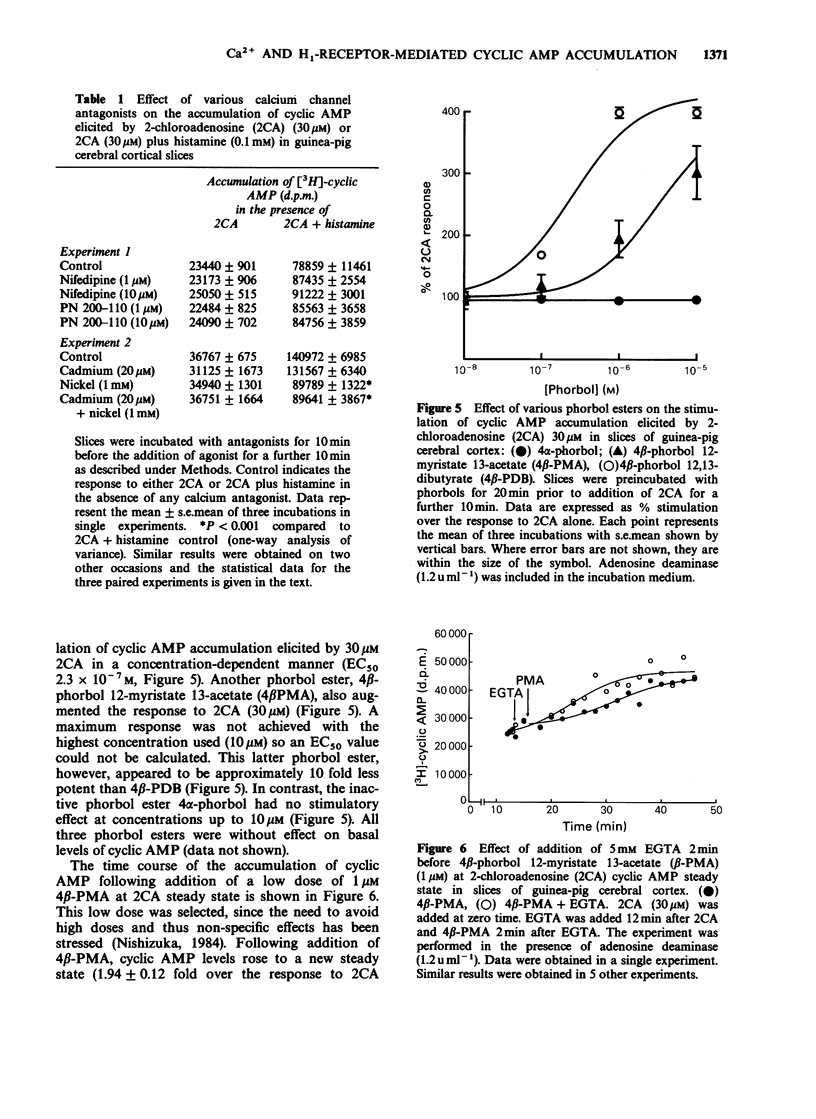
1. 2-Chloroadenosine (2CA) causes a maintained rise in adenosine 3':5'-cyclic monophosphate (cyclic AMP) content of guinea-pig cerebral cortical slices which is augmented by addition of histamine. We have investigated the temporal profile of the sensitivity of this response to calcium. 2. Rapid removal of extracellular calcium with EGTA (5 mM) at 2CA (30 microM)-induced steady state caused a slight increase in the cyclic AMP response to 2CA alone and completely abolished the augmentation produced by histamine (0.1 mM) added 20 min later. When EGTA was added only 2 min before histamine, the augmentation was reduced by 72%. 3. The calcium sensitivity of the histamine response was also indicated in studies in which EGTA was added 1 or 3 min after histamine at 2CA-induced steady state. Following addition of EGTA at either of these times, the augmentation was not maintained. 4. When calcium was rapidly removed with EGTA once a steady state level of cyclic AMP had been achieved with histamine, the augmentation response was maintained. This was despite the fact that EGTA had a similar effect on both extracellular free calcium and tissue calcium content when it was applied before or after histamine. 5. The 2CA response was augmented by phorbol esters (which mimic the actions of diacylglycerol) in a calcium-independent manner. 6. These results suggest that calcium is important for the initiation and early stages of the histamine-induced augmentation response. The apparent lack of calcium sensitivity of the response at later stages could mean that calcium is not involved in the maintenance of the response or that the intracellular machinery involved in the augmentation process becomes more sensitive to calcium as the response progresses, such that it becomes able to operate at a much lower level of intracellular calcium. A possible role for diacylglycerol in the maintenance of the response is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Gadi M., Hill S. J. The role of calcium in the cyclic AMP response to histamine in rabbit cerebral cortical slices. Br J Pharmacol. 1987 May;91(1):213–222. doi: 10.1111/j.1476-5381.1987.tb09001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruschi G., Bruschi M. E., Regolisti G., Borghetti A. Myoplasmic Ca2+-force relationship studied with fura-2 during stimulation of rat aortic smooth muscle. Am J Physiol. 1988 May;254(5 Pt 2):H840–H854. doi: 10.1152/ajpheart.1988.254.5.H840. [DOI] [PubMed] [Google Scholar]
- Carter T. D., Hallam T. J., Cusack N. J., Pearson J. D. Regulation of P2y-purinoceptor-mediated prostacyclin release from human endothelial cells by cytoplasmic calcium concentration. Br J Pharmacol. 1988 Dec;95(4):1181–1190. doi: 10.1111/j.1476-5381.1988.tb11754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claro E., Arbonés L., García A., Picatoste F. Phosphoinositide hydrolysis mediated by histamine H1-receptors in rat brain cortex. Eur J Pharmacol. 1986 Apr 16;123(2):187–196. doi: 10.1016/0014-2999(86)90659-x. [DOI] [PubMed] [Google Scholar]
- Coupet J., Szuchs-Myers V. A. Brain histamine H1- and H2-receptors and histamine-sensitive adenylate cyclase: effects of antipsychotics and antidepressants. Eur J Pharmacol. 1981 Sep 11;74(2-3):149–155. doi: 10.1016/0014-2999(81)90525-2. [DOI] [PubMed] [Google Scholar]
- Danoff S. K., Young J. M. Is histamine potentiation of adenosine-stimulated cyclic AMP accumulation in guinea-pig cerebral cortical slices mediated by products of inositol phospholipid breakdown? Biochem Pharmacol. 1987 Apr 1;36(7):1177–1179. doi: 10.1016/0006-2952(87)90432-1. [DOI] [PubMed] [Google Scholar]
- Daum P. R., Downes C. P., Young J. M. Histamine stimulation of inositol 1-phosphate accumulation in lithium-treated slices from regions of guinea pig brain. J Neurochem. 1984 Jul;43(1):25–32. doi: 10.1111/j.1471-4159.1984.tb06674.x. [DOI] [PubMed] [Google Scholar]
- Donaldson J., Brown A. M., Hill S. J. Influence of rolipram on the cyclic 3',5'-adenosine monophosphate response to histamine and adenosine in slices of guinea-pig cerebral cortex. Biochem Pharmacol. 1988 Feb 15;37(4):715–723. doi: 10.1016/0006-2952(88)90146-3. [DOI] [PubMed] [Google Scholar]
- Donaldson J., Hill S. J., Brown A. M. Kinetic studies on the mechanism by which histamine H1 receptors potentiate cyclic AMP accumulation in guinea pig cerebral cortical slices. Mol Pharmacol. 1988 Jun;33(6):626–633. [PubMed] [Google Scholar]
- Donaldson J., Hill S. J. Histamine-induced hydrolysis of polyphosphoinositides in guinea-pig ileum and brain. Eur J Pharmacol. 1986 May 27;124(3):255–265. doi: 10.1016/0014-2999(86)90226-8. [DOI] [PubMed] [Google Scholar]
- Garbarg M., Schwartz J. C. Synergism between histamine H1- and H2-receptors in the cAMP response in guinea pig brain slices: effects of phorbol esters and calcium. Mol Pharmacol. 1988 Jan;33(1):38–43. [PubMed] [Google Scholar]
- Green J. P., Johnson C. L., Weinstein H., Maayani S. Antagonism of histamine-activated adenylate cyclase in brain by D-lysergic acid diethylamide. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5697–5701. doi: 10.1073/pnas.74.12.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Influx of bivalent cations can be independent of receptor stimulation in human endothelial cells. Biochem J. 1989 Apr 1;259(1):125–129. doi: 10.1042/bj2590125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegstrand L. R., Kanof P. D., Greengard P. Histamine-sensitive adenylate cyclase in mammalian brain. Nature. 1976 Mar 11;260(5547):163–165. doi: 10.1038/260163a0. [DOI] [PubMed] [Google Scholar]
- Hill S. J., Daum P., Young J. M. Affinities of histamine H1-antagonists in guinea pig brain: similarity of values determined from [3H]mepyramine binding and from inhibition of a functional response. J Neurochem. 1981 Nov;37(5):1357–1360. doi: 10.1111/j.1471-4159.1981.tb04692.x. [DOI] [PubMed] [Google Scholar]
- Hill S. J. Histamine receptors in the mammalian central nervous system: biochemical studies. Prog Med Chem. 1987;24:29–84. doi: 10.1016/s0079-6468(08)70419-3. [DOI] [PubMed] [Google Scholar]
- Hill S. J., Kendall D. A. Cross-talk between different receptor-effector systems in the mammalian CNS. Cell Signal. 1989;1(2):135–141. doi: 10.1016/0898-6568(89)90002-8. [DOI] [PubMed] [Google Scholar]
- Hollingsworth E. B., Daly J. W. Accumulation of inositol phosphates and cyclic AMP in guinea-pig cerebral cortical preparations. Effects of norepinephrine, histamine, carbamylcholine and 2-chloroadenosine. Biochim Biophys Acta. 1985 Nov 20;847(2):207–216. doi: 10.1016/0167-4889(85)90022-9. [DOI] [PubMed] [Google Scholar]
- Hollingsworth E. B., Sears E. B., Daly J. W. An activator of protein kinase C (phorbol-12-myristate-13-acetate) augments 2-chloroadenosine-elicited accumulation of cyclic AMP in guinea pig cerebral cortical particulate preparations. FEBS Lett. 1985 May 20;184(2):339–342. doi: 10.1016/0014-5793(85)80634-7. [DOI] [PubMed] [Google Scholar]
- Kotlikoff M. I., Murray R. K., Reynolds E. E. Histamine-induced calcium release and phorbol antagonism in cultured airway smooth muscle cells. Am J Physiol. 1987 Oct;253(4 Pt 1):C561–C566. doi: 10.1152/ajpcell.1987.253.4.C561. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Magistretti P. J., Schorderet M. Norepinephrine and histamine potentiate the increases in cyclic adenosine 3':5'-monophosphate elicited by vasoactive intestinal polypeptide in mouse cerebral cortical slices: mediation by alpha 1-adrenergic and H1-histaminergic receptors. J Neurosci. 1985 Feb;5(2):362–368. doi: 10.1523/JNEUROSCI.05-02-00362.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Kanaide H., Nishimura J., Shogakiuchi Y., Kobayashi S., Nakamura M. Histamine activates H1-receptors to induce cytosolic free calcium transients in cultured vascular smooth muscle cells from rat aorta. Biochem Biophys Res Commun. 1986 Feb 26;135(1):172–177. doi: 10.1016/0006-291x(86)90958-7. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Palacios J. M., Garbarg M., Barbin G., Schwartz J. C. Pharmacological characterization of histamine receptors mediating the stimulation of cyclic AMP accumulation in slices from guinea-pig hippocampus. Mol Pharmacol. 1978 Nov;14(6):971–982. [PubMed] [Google Scholar]
- Park S., Rasmussen H. Activation of tracheal smooth muscle contraction: synergism between Ca2+ and activators of protein kinase C. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8835–8839. doi: 10.1073/pnas.82.24.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Steinhardt R., Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986 Aug 22;233(4766):886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- Reynolds E. E., Dubyak G. R. Agonist-induced calcium transients in cultured smooth muscle cells: measurements with fura-2 loaded monolayers. Biochem Biophys Res Commun. 1986 May 14;136(3):927–934. doi: 10.1016/0006-291x(86)90421-3. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Ohga Y., Daly J. W. The role of calcium in the regulation of cyclic nucleotide levels in brain slices of rat and guinea pig. Naunyn Schmiedebergs Arch Pharmacol. 1978 Apr;302(2):141–151. doi: 10.1007/BF00517981. [DOI] [PubMed] [Google Scholar]
- Shonk R. F., Rall T. W. Ontogeny of adenosine 3',5'-monophosphate metabolism in guinea pig cerebral cortex. I. Development of responses to histamine, norepinephrine and adenosine. Mol Cell Biochem. 1987 Feb;73(2):141–155. doi: 10.1007/BF00219428. [DOI] [PubMed] [Google Scholar]
- Takuwa Y., Takuwa N., Rasmussen H. Measurement of cytoplasmic free Ca2+ concentration in bovine tracheal smooth muscle using aequorin. Am J Physiol. 1987 Dec;253(6 Pt 1):C817–C827. doi: 10.1152/ajpcell.1987.253.6.C817. [DOI] [PubMed] [Google Scholar]
- Triggle D. J., Janis R. A. Calcium channel ligands. Annu Rev Pharmacol Toxicol. 1987;27:347–369. doi: 10.1146/annurev.pa.27.040187.002023. [DOI] [PubMed] [Google Scholar]
- Yoshimasa T., Sibley D. R., Bouvier M., Lefkowitz R. J., Caron M. G. Cross-talk between cellular signalling pathways suggested by phorbol-ester-induced adenylate cyclase phosphorylation. Nature. 1987 May 7;327(6117):67–70. doi: 10.1038/327067a0. [DOI] [PubMed] [Google Scholar]


