Abstract
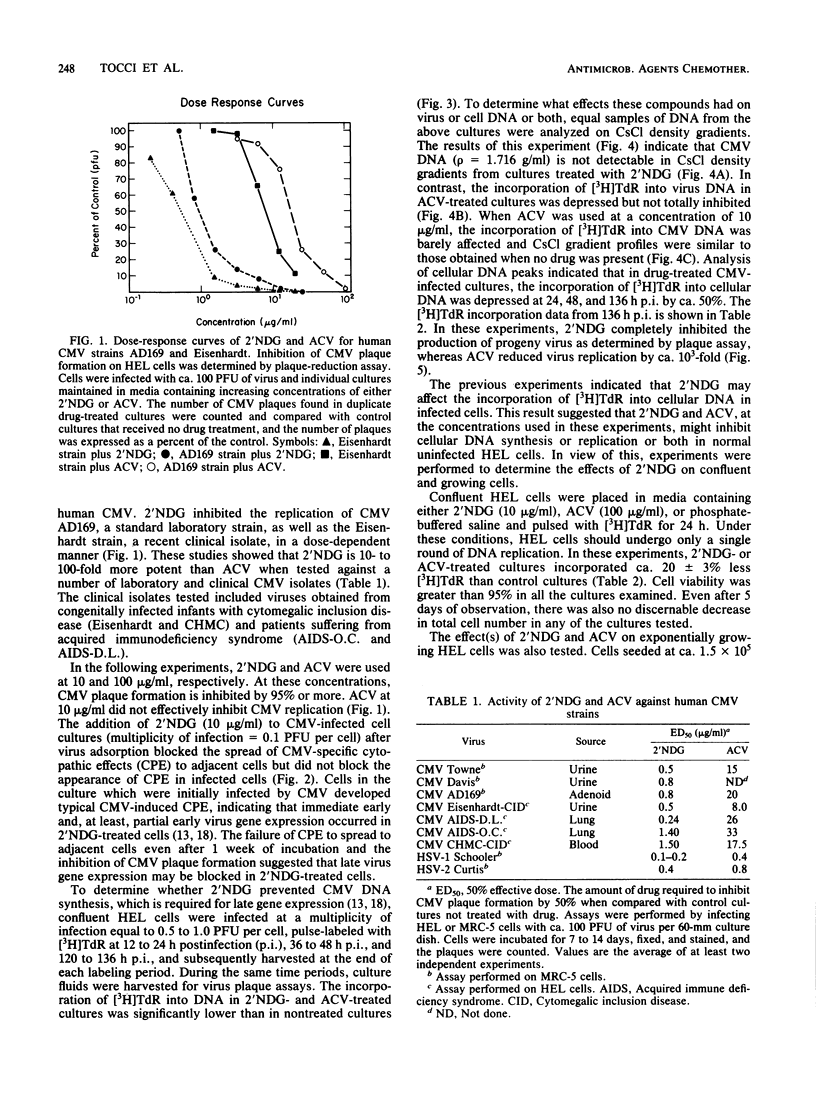
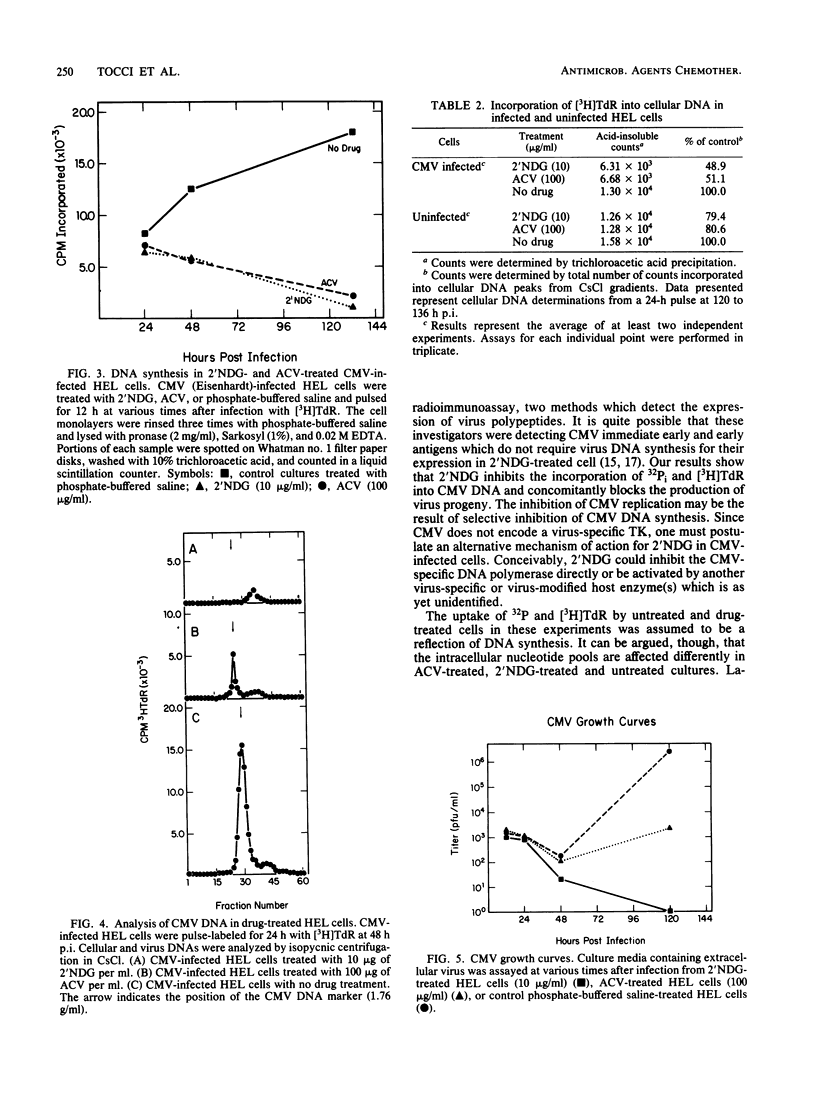
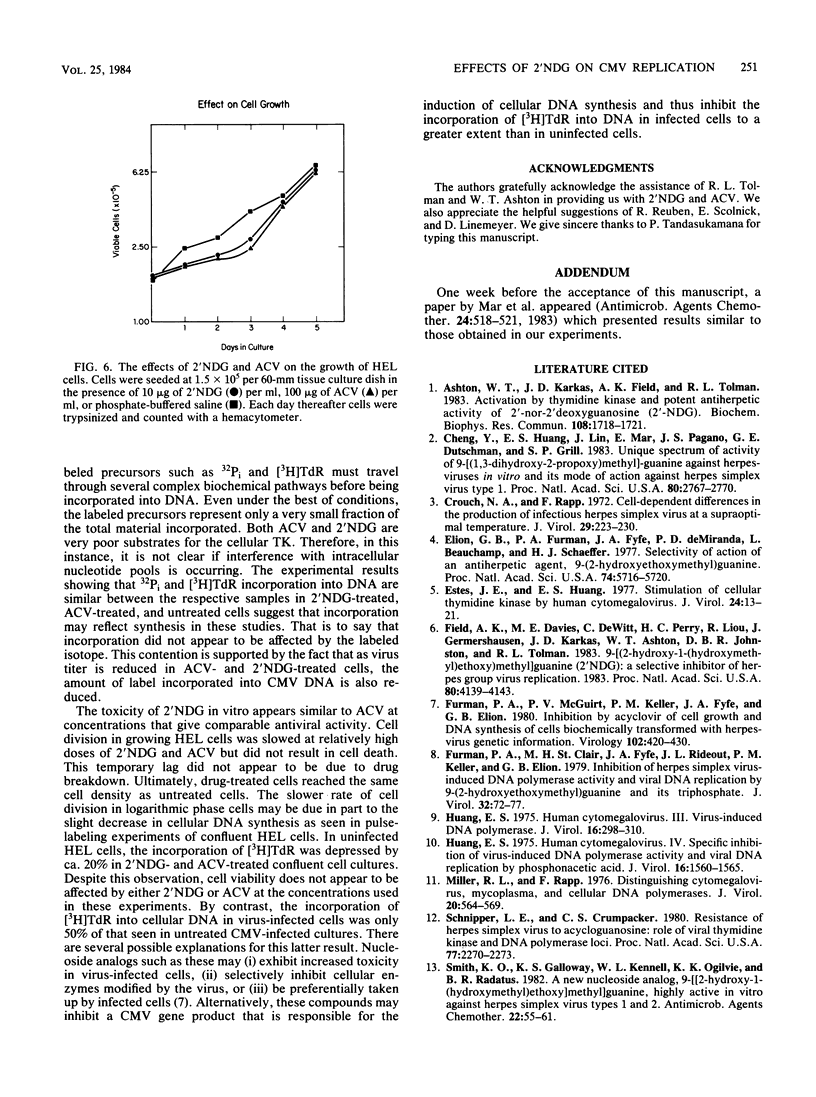
The nucleoside analog 2'-nor-2'-deoxyguanosine (2'NDG) effectively inhibits the replication of several laboratory and clinical isolates of human cytomegalovirus. These isolates included viruses obtained from congenitally infected infants and patients suffering from acquired immune deficiency syndrome. The dose of 2'NDG that inhibited cytomegalovirus plaque formation ranged from 0.1 to 1.6 micrograms/ml. At 10 micrograms/ml, 2'NDG completely blocked the production of virus progeny but not the expression of immediate early and early virus gene functions. Cytomegalovirus DNA was not detectable in 2'NDG-treated virus-infected human embryo lung cells when assayed by CsCl density gradient centrifugation. In contrast, the guanosine analog acyclovir at 100 micrograms/ml did not inhibit the production of virus or the synthesis of cytomegalovirus DNA. In virus-infected cells, 2'NDG and acyclovir at 10 and 100 micrograms/ml, respectively, inhibited the incorporation of [3H]thymidine and 32Pi into cellular DNA by ca. 50%. Uninfected human embryo lung cells grown in these concentrations of acyclovir or 2'NDG exhibited a slightly transient lag phase but, overall, cell growth was not retarded, and there was no decrease in cell viability. The extended lag in cell division was not due to inactivation or breakdown of the antiviral compounds but may be due in part to a temporary decrease in cellular DNA synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton W. T., Karkas J. D., Field A. K., Tolman R. L. Activation by thymidine kinase and potent antiherpetic activity of 2'-nor-2'-deoxyguanosine (2'NDG). Biochem Biophys Res Commun. 1982 Oct 29;108(4):1716–1721. doi: 10.1016/s0006-291x(82)80109-5. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Huang E. S., Lin J. C., Mar E. C., Pagano J. S., Dutschman G. E., Grill S. P. Unique spectrum of activity of 9-[(1,3-dihydroxy-2-propoxy)methyl]-guanine against herpesviruses in vitro and its mode of action against herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1983 May;80(9):2767–2770. doi: 10.1073/pnas.80.9.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch N. A., Rapp F. Cell-dependent differences in the production of infectious herpes simplex virus at a supraoptimal temperature. J Virol. 1972 Feb;9(2):223–230. doi: 10.1128/jvi.9.2.223-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes J. E., Huang E. S. Stimulation of cellular thymidine kinases by human cytomegalovirus. J Virol. 1977 Oct;24(1):13–21. doi: 10.1128/jvi.24.1.13-21.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. K., Davies M. E., DeWitt C., Perry H. C., Liou R., Germershausen J., Karkas J. D., Ashton W. T., Johnston D. B., Tolman R. L. 9-([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine: a selective inhibitor of herpes group virus replication. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4139–4143. doi: 10.1073/pnas.80.13.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., McGuirt P. V., Keller P. M., Fyfe J. A., Elion G. B. Inhibition by acyclovir of cell growth and DNA synthesis of cells biochemically transformed with herpesvirus genetic information. Virology. 1980 Apr 30;102(2):420–430. doi: 10.1016/0042-6822(80)90109-9. [DOI] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Fyfe J. A., Rideout J. L., Keller P. M., Elion G. B. Inhibition of herpes simplex virus-induced DNA polymerase activity and viral DNA replication by 9-(2-hydroxyethoxymethyl)guanine and its triphosphate. J Virol. 1979 Oct;32(1):72–77. doi: 10.1128/jvi.32.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. III. Virus-induced DNA polymerase. J Virol. 1975 Aug;16(2):298–310. doi: 10.1128/jvi.16.2.298-310.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. IV. Specific inhibition of virus-induced DNA polymerase activity and viral DNA replication by phosphonoacetic acid. J Virol. 1975 Dec;16(6):1560–1565. doi: 10.1128/jvi.16.6.1560-1565.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeor S. C., Albrecht T. B., Funk F. D., Rapp F. Stimulation of cellular DNA synthesis by human cytomegalovirus. J Virol. 1974 Feb;13(2):353–362. doi: 10.1128/jvi.13.2.353-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar E. C., Cheng Y. C., Huang E. S. Effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine on human cytomegalovirus replication in vitro. Antimicrob Agents Chemother. 1983 Oct;24(4):518–521. doi: 10.1128/aac.24.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. L., Rapp F. Distinguishing cytomegalovirus, mycoplasma, and cellular DNA polymerases. J Virol. 1976 Dec;20(3):564–569. doi: 10.1128/jvi.20.3.564-569.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnipper L. E., Crumpacker C. S. Resistance of herpes simplex virus to acycloguanosine: role of viral thymidine kinase and DNA polymerase loci. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2270–2273. doi: 10.1073/pnas.77.4.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. O., Galloway K. S., Kennell W. L., Ogilvie K. K., Radatus B. K. A new nucleoside analog, 9-[[2-hydroxy-1-(hydroxymethyl)ethoxyl]methyl]guanine, highly active in vitro against herpes simplex virus types 1 and 2. Antimicrob Agents Chemother. 1982 Jul;22(1):55–61. doi: 10.1128/aac.22.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair M. H., Furman P. A., Lubbers C. M., Elion G. B. Inhibition of cellular alpha and virally induced deoxyribonucleic acid polymerases by the triphosphate of acyclovir. Antimicrob Agents Chemother. 1980 Nov;18(5):741–745. doi: 10.1128/aac.18.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jeor S., Rapp F. Cytomegalovirus replication in cells pretreated with 5-iodo-2'-deoxyuridine. J Virol. 1973 Jun;11(6):986–990. doi: 10.1128/jvi.11.6.986-990.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F. Sequence of protein synthesis in cells infected by human cytomegalovirus: early and late virus-induced polypeptides. J Virol. 1978 Jun;26(3):686–701. doi: 10.1128/jvi.26.3.686-701.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth B. B., French L. Plaque assay of cytomegalovirus strains of human origin. Proc Soc Exp Biol Med. 1970 Nov;135(2):253–258. doi: 10.3181/00379727-135-35031. [DOI] [PubMed] [Google Scholar]
- Závada V., Erban V., Rezácová D., Vonka V. Thymidine-kinase in cytomegalovirus infected cells. Arch Virol. 1976;52(4):333–339. doi: 10.1007/BF01315622. [DOI] [PubMed] [Google Scholar]



