Abstract
When engaged by a stimulus, different nodes of a neural circuit respond in a coordinated fashion. We often ask whether there is a cause and effect in such interregional interactions. This paper proposes that we can infer causality in functional connectivity by employing a ‘perturb and measure’ approach. In the human brain, this has been achieved by combining transcranial magnetic stimulation (TMS) with positron emission tomography (PET), functional magnetic resonance imaging or electroencephalography. Here, I will illustrate this approach by reviewing some of our TMS/PET work, and will conclude by discussing a few methodological and theoretical challenges facing those studying neural connectivity using a perturbation.
Keywords: transcranial magnetic stimulation, positron emission tomography, neural connectivity, cerebral cortex
1. Introduction
Over the past decade, functional neuroimaging moved from mapping various cognitive, perceptual and motor processes onto discrete brain regions towards exploring functional interactions between specialized modules. The primary tool in this endeavour has been a statistical analysis of changes in neural activity, indexed by regional cerebral blood flow or blood oxygenation-level dependent (BOLD) signal, measured during the subject's performance of various tasks. Such analyses provide a wealth of information about a coordinated neural response to a given stimulus throughout the brain. It has become obvious, however, that the current neuroimaging methods do not allow us to discern the nature of such interregional interactions vis-à-vis their possible causality. For example, if a given stimulus ‘activates’ regions A, B and C in a coordinated fashion, we do not know whether region A influences region B, or region C drives both regions A and B with no direct interactions taking place between A and B. Several contributions in this issue address this problem at a mathematical level (Eichler 2005; Penny et al. 2005; Harrison et al. 2005). Many evoke so-called Granger causality, which is based on the notion that an effect cannot precede its cause in time (Granger 1969). Given the poor temporal resolution of positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), our ability to infer Granger causality in brain images is limited. Here, an alternative approach is reviewed; namely, the use of physical perturbation of neural activity to evaluate possible cause and effect in the context of neural connectivity.
2. Perturbation
Perturbation as a tool for studying brain–behaviour relationships has a long history. Irreversible perturbations (i.e. brain lesions) told us, for example, that the inferior frontal cortex is essential for language production (Broca 1861) and the hippocampal system for declarative memory (Scoville & Milner 1957). Reversible perturbation of neural activity with direct electrical stimulation revealed the somatotopic organization of the motor cortex (Fritsch & Hitzig 1870; Leyton & Sherington 1917; Penfield & Rasmussen 1950). More recently, reversible inactivation of specific brain regions could be achieved in experimental animals with local cooling or by microinjections of local anaesthetics and agonists/antagonists of neurotransmitters (reviewed in Payne et al. 1996; Martin & Ghez 1999). In these (and other similar) studies, changes in neural activity have been recorded at the site of perturbation. Intracortical microstimulation has also been used, for example, to ‘activate’ briefly distinct populations of neurons in motion-sensitive cortical areas biasing, in turn, the monkey's perception of visual motion (reviewed in Cohen & Newsome 2004).
Beyond the local effects, several investigators explored consequences of perturbing one region on neural activity measured in adjacent or distal regions. Using a neural network with connectivity derived from experimental data, Kotter & Sommer (2000) demonstrated that the pattern of anatomic connectivity predicts cortical propagation of activity significantly better than networks with different types of neighbourhood-based connectivity or random connections. Experimentally, the role of feedback connections within the visual cortex has been studied by perturbing (with local cooling or GABA microinjections) activity in one area (e.g. MT or V2) on receptive-field neuronal properties in another area (e.g. V1; Angelucci & Bullier 2003). It is of note that such feedback effects are very fast (less than 10 ms) and are therefore unlikely to provide sufficient temporal separation for statistical approaches based on the Granger causality in most human studies. Another example of the unique insights gained by combining local perturbations with the assessment of their consequences on neural activity is the work by Vanduffel et al. (1997), who inactivated the middle suprasylvian (visual) cortex of the cat by cooling and measured neural activity indirectly with 2-deoxyglucose throughout the brain. Compared with the strengths of the anatomic connections, cooling-induced decreases in metabolic activity were found to be stronger upstream than downstream of the inactivated region. For example, cooling effects in the cingulate cortex and the primary visual cortex were greater and smaller, respectively, than predicted from the density of anatomic connections (Vanduffel et al. 1997). It was also observed that feed-forward effects extended beyond one synapse and also affected regions not directly connected with the cooled cortex. These two examples illustrate the power of the perturb and record approach—one in the real-time assessments of neural connectivity between two sites and the other of the connectivity between one site and the rest of the brain. The latter approach is now described in the context of studies of neural connectivity in the human brain.
3. TMS and PET
During the 1990s, we (Paus et al. 1997) and others (Fox et al. 1997; Siebner et al. 1998) applied the above perturb and record approach in order to map neural connections in the living human brain. This method combines two well-established tools of brain research: transcranial magnetic stimulation (TMS) and PET. TMS is used to stimulate directly a selected cortical area while simultaneously measuring changes in brain activity with PET. At about the same time, other investigators combined—for the same purpose—TMS with electroencephalography (EEG; Ilmoniemi et al. 1997) and fMRI (Bohning et al. 1998). Since the initial reports, this approach has been applied in a number of studies of neural excitability and connectivity of various regions of the human frontal cortex (reviewed in Paus 2002). Rather than reviewing this growing body of literature, the focus here will be on some basic principles of the combined TMS/PET method and its limitations.
TMS uses a time-varying magnetic field to induce, through the skull, electrical current in a spatially restricted region of the cerebral cortex. TMS can be used in three different modes: (i) single-pulse TMS (>∼4 s interval between two pulses), (ii) short trains of high-frequency TMS (e.g. 1 s train of 10 Hz stimulation); and (iii) long trains of low-frequency TMS (e.g. 15 min train of 1 Hz stimulation). The nature of the perturbations induced by TMS depends on the stimulation mode.
At a behavioural level, single pulses can elicit an overt response, a muscle twitch when stimulating the motor cortex or a phosphene when applied over the visual cortex, or modulate excitability of the stimulated region. Modulatory effects are relatively brief (tens of milliseconds), and could either increase or decrease responsiveness of the stimulated tissue to the subsequent stimulus. In the motor cortex, for example, ‘intracortical’ inhibition and excitation are assessed using the paired-pulse paradigm, whereby two TMS pulses are applied in rapid succession (1–30 ms between-pulse interval). Paired-pulse TMS with short (1–5 ms) and long (8–30 ms) between-pulse intervals elicits suppression and facilitation, respectively, of the muscle response (Kujirai et al. 1993; Ziemann et al. 1996a; Nakamura et al. 1997). Early suppression and later facilitation suggest somewhat different dynamics of inhibitory and excitatory processes activated by TMS in the motor cortex under these conditions. Pharmacological (Ziemann et al. 1996b; Werhahn et al. 1999) and in vitro (Avoli et al. 1997) studies suggest that the short (paired-pulse) and long (silent period) lasting cortical inhibition is mediated by GABA-A and GABA-B receptors, respectively. It appears, however, that local and distal blood-flow response to the paired-pulse stimulation correlates positively with the amount of modulation regardless of its direction (Strafella & Paus 2001). This observation suggests that the conditioning stimulus might have induced the release of glutamate that activated both excitatory and inhibitory interneurons; glutamate release is known to be associated with the production of nitric oxide and, in turn, with local increases in blood flow (reviewed in Paus 2002).
Effects of single-pulse TMS in non-motor regions are less well understood. Although single-pulse TMS is typically used to interfere with ongoing neuronal activity in the stimulated region (‘virtual lesions’; reviewed in Pascual-Leone et al. 2000), we have seen facilitation of visual attention (Grosbras & Paus 2002) and visual awareness (Grosbras & Paus 2003) shortly (50 ms) after single-pulse stimulation of the frontal eye field (FEF). These latter findings were explained as mediated by either local (i.e. FEF) increases in cortical excitability or feedback modulation of extrastriate visual areas. Other examples of distal effects of single-pulse TMS include transcallosal modulation of cortico-spinal excitability (Chen et al. 2003) and interactions between primary and secondary visual cortex (Pascual-Leone & Walsh 2001).
Although single-pulse TMS has the advantage of having high temporal resolution and could thus provide information not only about where but also when a perturbation affects neural activity, the poor temporal resolution of PET and fMRI does not allow us to measure neural consequences of a single TMS pulse. At present, even TMS-compatible EEG appears to be inadequate for capturing short-latency (<20 ms) neural responses; several laboratories are working on overcoming this limitation.
(a) Short-trains of high-frequency TMS
Repetitive TMS provide a more robust way of perturbing neural activity, which are detectable with PET and fMRI. Typically, high-frequency TMS applied over the motor cortex increases cortico-spinal excitability (Chen 2000). In our first TMS/PET study (Paus et al. 1997), we used a parametric design and, during each scan, applied a different number of trains of 10 Hz stimulation over the FEF; the distal response was observed in a subset of cortical regions known to be connected to the FEF in the cerebral cortex of non-human primates. In subsequent studies, we used a somewhat different approach and applied a series of 10 Hz trains between scans to modulate neural activity of the mid-dorsolateral frontal cortex (MDL-FC; Paus et al. 2001; Barrett et al. 2004). In these experiments, we observed a robust blood-flow response in brain regions presumably connected with the stimulation site, such as the anterior cingulate cortex and the caudate nucleus. Using direct electrical stimulation of the frontal cortex, we have also confirmed the presence of similar changes in the fronto-cingulate connectivity in an anaesthetized rat (Paus et al. 2001).
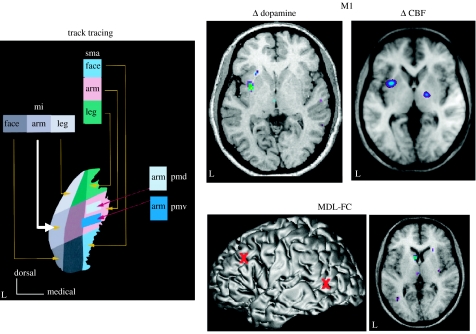
Moving beyond the indirect assessment of neural activity with 15O-H2O, we used 11C-raclopride to assess TMS-induced changes in dopamine release in the human striatum. When stimulating the left MDL-FC, a decrease in the 11C-raclopride binding potential was observed, indicating an increase in local release of endogenous dopamine, in the left caudate nucleus (Strafella et al. 2001). On the other hand, TMS applied over the left primary motor cortex resulted in a decrease in binding potential (increase in dopamine release) in the left putamen (Strafella et al. 2003). As illustrated in figure 1, these results are consistent with the known cortico-striatal connectivity in the monkey; namely, the existence of efferent connections from the prefrontal and motor cortices to the caudate nucleus and the putamen, respectively. We believe that cortical stimulation led to an ‘activation’ of specific cortico-striatal glutamatergic pathways, which, in turn, induced local release of dopamine at their terminations in the striatum.
Figure 1.
Dopamine release induced by repetitive TMS of the frontal cortex. Left: organization of cortico-striatal projections to the monkey putamen (from Takada et al. 1998). Top right: changes in dopamine release (from Strafella et al. 2003) and blood flow (from Chouinard et al. 2003) in the human putamen following rTMS applied over the left primary motor cortex (M1). Bottom right: changes in dopamine release (from Strafella et al. 2001) in the human caudate nucleus following rTMS applied over the left mid-dorsolateral frontal cortex (MDLFC). Location (red markers) of the two stimulation sites, the left MDLFC and the left occipital (OCC) cortex, on the MRI of one subject in stereotaxic space. Reprinted with permission from Paus & Barrett (2004).
(b) Long-trains of low-frequency TMS
Low frequency TMS is often used to decrease excitability of the stimulated neural system (Chen 2000; Hilgetag et al. 2001; MacDonald & Paus 2003). Such stimulation has not only local effects (Chen et al. 2003; Maeda et al. 2000; Muellbacher et al. 2000) but can also affect distal sites. This has been demonstrated behaviourally by measuring motor evoked potentials (MEPs) elicited by single-pulse TMS applied over the primary motor cortex; MEPs are reduced after low-frequency (1 Hz) stimulation of the premotor cortex, presumably through direct cortico-cortical connections (Gerschlager et al. 2001). Using PET, several investigators applied low-frequency TMS over the primary motor and premotor cortices to examine their connectivity (Siebner et al. 1998, 2000, 2001; Chouinard et al. 2003). It has been shown that low-frequency (1 Hz, 2×15 min) TMS applied over the primary motor cortex induced changes in MEPs that correlated with those in blood flow in a limited set of brain regions presumably connected with the stimulation site, including the contralateral primary motor cortex, the putamen and the cerebellum (Chouinard et al. 2003). When the same stimulation was applied over the dorsal premotor cortex, again, changes in MEPs were observed but this time, they correlated with blood-flow response in a different and more extended set of regions, including the ventral premotor cortex, supplementary motor area, mid-dorsolateral and mid-ventrolateral frontal cortex, and the superior parietal lobule (Chouinard et al. 2003). These findings are again consistent with the known anatomic connectivity of the primary motor and dorsal premotor cortices in the monkey (Parent & Hazrati 1995; Matelli & Luppino 1997, 2000). In these and other TMS/PET studies, however, significant blood-flow response is observed only in a subset of regions known to possess anatomic connections in the monkey homologue of the stimulated cortical site. A few possible reasons for this discrepancy will be discussed in the following section.
4. Challenges
The most obvious reason for revealing only a subset of regions with known anatomic connections with the stimulation site is statistical power; given the complexity of the experimental set up, TMS/PET studies typically include only six to eight subjects. Another possible factor, common to all neuroimaging studies, is interindividual variability in the exact location of a given cortical area and its connectivity (e.g. MacNeil et al. 1997; Hilgetag & Grant 2000). For some regions, this may not be an issue. For example, the physical location of the primary motor cortex is fairly invariable across individuals and, furthermore, correct coil location can be simply verified by the presence of a behavioural response to TMS, i.e. a muscle twitch. But other regions, such as the MDL-FC, may be more variable in their location and, at the same time, are less easy to verify functionally. A standard ‘functional probe’ known to engage the MDL-FC, such as a working memory task, may give different locations in different subjects for two reasons: (i) the same functional area is located in a different part of the prefrontal cortex; and (ii) different (albeit related) cortical area was engaged, perhaps owing to different ways the same task was performed by the subjects. In a group of subjects, do we position the coil over the same anatomic location, or do we vary the coil location based on the outcome of a functional scout? In the past, both strategies have been used in different studies (anatomic locations: e.g. Paus et al. 1997, 2001; Strafella et al. 2001; Chouinard et al. 2003; functional scout: e.g. Rushworth et al. 2002; Köhler et al. 2004) but whether or not they may result in different outcomes has never been evaluated. If we take the pattern of cortico-cortical connectivity as one of the main defining features of a given region, then the TMS/PET approach may be ideal for answering this question. The prediction is simple: stimulation of the ‘true’ MDL-FC would yield a more robust, or a more plausible pattern of neural connectivity.
The final and theoretically most interesting issue is that of possible biological reasons for differences between anatomic and ‘perturb and record’ connectivity estimates. Recall the findings of Vanduffel et al. (1997), in which distal effects of cooling a particular cortical region did not match the density of anatomic connections in their magnitude and extended beyond one synapse. Clearly, the perturbation modulates neural activity differently in the different nodes of the same circuit. We can only speculate about the possible reasons. In the rat visual system, for example, ultra-structural studies show that the number of inputs on GABAergic interneurons is significantly lower for feedback, as compared with feed-forward, connections (Johnson & Burkhalter 1996). This observation would suggest a relative absence of inhibition in feedback, relative to feed-forward, pathways. Indeed, this prediction was confirmed by neurophysiological studies carried out in slices of rat visual cortex (Shao & Burkhalter 1996, 1999) and is consistent with the Vanduffel et al. findings. Another important variable to consider is the rate of spontaneous neural activity in different nodes of the stimulated circuit during the recordings. As single-unit recordings make abundantly clear, cortical neurons continue to fire even in the absence of a specific input/output or task requirement; the rate of spontaneous firing varies from region to region but, on average, often exceeds 10 spikes s−1 (Schall, personal communication). In the case of a 60 s scan, this amounts to a total of 600 spikes per neuron. With an estimated 50 million neurons cm−3 of cortex (Pakkenberg & Gundersen 1997), even if only 0.01% of all neurons discharged at any given time, a total of 3 million spikes could be generated within a single resolution element of the PET image and affect post-synaptic neurotransmission in another resolution element. It is probable that the local and distal effects of high- or low-frequency TMS applied over a given cortical region would be a product of an interaction between the rate of spontaneous activity in the different nodes of the stimulated circuit and the type of local circuitry. In turn, these may vary as a function of the subject's state during the experiment, and also as a function of long-term changes in local-circuit structural or functional properties, such as those induced by learning or a disease. While the former factor represents a potential confounder and should be controlled as much as possible, the latter examples illustrate the potential of the ‘perturb and record’ approach in studying modifications of neural connectivity in healthy and disordered human brain.
Acknowledgments
I thank my colleagues at the Montreal Neurological Institute for their contributions to the ongoing TMS/PET studies of cortical excitability and connectivity. The author's research is supported by the Canadian Institute of Health Research, the Canadian Foundation for Innovation and the National Science and Engineering Research Council of Canada.
Footnotes
One contribution of 21 to a Theme Issue ‘Multimodal neuroimaging of brain connectivity’.
References
- Angelucci A, Bullier J. Reaching beyond the classical receptive field of V1 neurons: horizontal or feedback axons? J. Physiol. Paris. 2003;97:141–154. doi: 10.1016/j.jphysparis.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Avoli M, Hwa G, Louvel J, Kurcewicz I, Pumain R, Lacaille J.C. Functional and pharmacological properties of GABA-mediated inhibition in the human neocortex. Can. J. Physiol. Pharmacol. 1997;75:526–534. [PubMed] [Google Scholar]
- Barrett J, Della-Maggiore V, Chouinard P, Paus T. Mechanisms of action underlying the effect of repetitive transcranial magnetic stimulation on mood: behavioral and imaging studies. Neuropsychopharmacology. 2004;29:1172–1189. doi: 10.1038/sj.npp.1300411. [DOI] [PubMed] [Google Scholar]
- Bohning D.E, Shastri A, Nahas Z, Lorberbaum J.P, Andersen S.W, Dannels W.R, Haxthausen E.U, Vincent D.J, George M.S. Echoplanar BOLD fMRI of brain activation induced by concurrent transcranial magnetic stimulation. Invest. Radiol. 1998;33:336–340. doi: 10.1097/00004424-199806000-00004. [DOI] [PubMed] [Google Scholar]
- Broca P. Nouvelle observation d'aphémie produite par une lésion de la moitié postérieure des deuxième et troisième circonvolution frontales gauches. Bulletin de la Société Anatomique, 1861d, tome. 1861;XXXVI:398–407. [Google Scholar]
- Chen R. Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve Suppl. 2000;9:S26–S32. doi: 10.1002/1097-4598(2000)999:9<::aid-mus6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li J.Y. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J. Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Chouinard P.A, Van der Werf Y.D, Leonard G, Paus T. Modulation of neural connectivity induced by low-frequency transcranial magnetic stimulation of the dorsal premotor and primary motor cortices: a TMS/PET study. J. Neurophysiol. 2003;90:1071–1083. doi: 10.1152/jn.01105.2002. [DOI] [PubMed] [Google Scholar]
- Cohen M.R, Newsome W.T. What electrical microstimulation has revealed about the neural basis of cognition. Curr. Opin. Neurobiol. 2004;14:169–177. doi: 10.1016/j.conb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Eichler M. A graphical approach for evaluating effective connectivity in neural systems. Phil. Trans. R. Soc. B. 2005;360:953–967. doi: 10.1098/rstb.2005.1641. doi:10.1098/rstb.2005.1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P, Ingham R, George M.S, Mayberg H, Ingham J, Roby J, Martin C, Jerabek P. Imaging intra-cerebral connectivity by PET during TMS. NeuroReport. 1997;8:2787–2791. doi: 10.1097/00001756-199708180-00027. [DOI] [PubMed] [Google Scholar]
- Fritsch G, Hitzig E. Über die elektrische Erragbarkeit des Grosshirns. Arch. Anat. Physiol. (Leipzig) 1870;37:300–332. [Google Scholar]
- Gerschlager W, Siebner H.R, Rothwell J.C. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology. 2001;57:449–455. doi: 10.1212/wnl.57.3.449. [DOI] [PubMed] [Google Scholar]
- Granger C.W.J. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37:424–438. [Google Scholar]
- Grosbras M.H, Paus T. Transcranial magnetic stimulation of the frontal eye-field: effects on visual perception and attention. J. Cogn. Neurosci. 2002;14:1109–1120. doi: 10.1162/089892902320474553. [DOI] [PubMed] [Google Scholar]
- Grosbras M.H, Paus T. Transcranial magnetic stimulation of the human frontal eye field facilitates visual awareness. Eur. J. Neurosci. 2003;18:3121–3126. doi: 10.1111/j.1460-9568.2003.03055.x. [DOI] [PubMed] [Google Scholar]
- Harrison L.M, David O, Friston K.J. Stochastic models of neuronal dynamics. Phil. Trans. R. Soc. B. 2005;360:1075–1091. doi: 10.1098/rstb.2005.1648. doi:10.1098/rstb.2005.1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgetag C.C, Grant S. Uniformity, specificity and variability of corticocortical connectivity. Phil. Trans. R. Soc. B. 2000;355:7–20. doi: 10.1098/rstb.2000.0546. doi:10.1098/rstb.2000.0546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgetag C.C, Theoret H, Pascual-Leone A. Enhanced visual spatial attention ipsilateral to rTMS-induced ‘virtual lesions’ of human parietal cortex. Nat. Neurosci. 2001;4:953–957. doi: 10.1038/nn0901-953. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi R.J, Virtanen J, Ruohonen J, Karhu J, Aronen H.J, Naatanen R, Katila T. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. NeuroReport. 1997;8:3537–3540. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- Johnson R.R, Burkhalter A. Microcircuitry of forward and feedback connections within rat visual cortex. J. Comp. Neurol. 1996;368:383–398. doi: 10.1002/(SICI)1096-9861(19960506)368:3<383::AID-CNE5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Köhler S, Paus T, Buckner R.L, Milner B. Effects of left inferior prefrontal stimulation on episodic memory formation: a two-stage fMRI-rTMS study. J. Cogn. Neurosci. 2004;16:178–188. doi: 10.1162/089892904322984490. [DOI] [PubMed] [Google Scholar]
- Kotter R, Sommer F.T. Global relationship between anatomical connectivity and activity propagation in the cerebral cortex. Phil. Trans. R. Soc. B. 2000;355:127–134. doi: 10.1098/rstb.2000.0553. doi:10.1098/rstb.2000.0553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia M.D, Rothwell J.C, Day B.L, Thompson P.D, Ferbert A, Wroe S, Asselman P, Marsden C.D. Corticocortical inhibition in human motor cortex. J. Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton A.S.F, Sherington C.S. Observations on the excitable cortex of the chimpanzee, orang-utan and gorilla. Q. J. Exp. Physiol. 1917;11:135–222. [Google Scholar]
- MacDonald P.A, Paus T. The role of parietal cortex in awareness of self-generated movements: a transcranial magnetic stimulation study. Cereb. Cortex. 2003;13:962–967. doi: 10.1093/cercor/13.9.962. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan J.P, Tormos J.M, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp. Brain Res. 2000;133:425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- Martin J.H, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J. Neurosci. Methods. 1999;86:145–159. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G. Functional anatomy of human motor cortical areas. In: Boller F, Grafman J, editors. Handbook of neuropsychology. vol. 11. Elsevier; Amsterdam, Netherlands: 1997. pp. 9–26. [Google Scholar]
- Matelli M, Luppino G. Parietofrontal circuits: parallel channels for sensory–motor integrations. In: Williamson P.D, Siegel A.M, Roberts D.W, Thadani V.M, Gazzaniga M.S, editors. Advances in neurology. vol. 84. Lippincott Williams & Wilkins; Philadelphia, PA: 2000. pp. 51–61. [PubMed] [Google Scholar]
- MacNeil M.A, Lomber S.G, Payne B.R. Thalamic and cortical projections to middle suprasylvian cortex of cats: constancy and variation. Exp. Brain Res. 1997;114:24–32. doi: 10.1007/pl00005620. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Hallett M. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin. Neurophysiol. 2000;111:1002–1007. doi: 10.1016/s1388-2457(00)00284-4. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J. Physiol. 1997;498:823–871. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen H.J. Neocortical neuron number in humans: effect of sex and age. J. Comp. Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Parent A, Hazrati L.N. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V. Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science. 2001;292:510–512. doi: 10.1126/science.1057099. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience—virtual lesion, chronometry, and functional connectivity. Curr. Opin. Neurobiol. 2000;10:232–237. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- Paus T. Combination of transcranial magnetic stimulation with brain imaging. In: Mazziotta J, Toga A, editors. Brain mapping: the methods. 2nd edn. Academic Press; New York: 2002. pp. 691–705. [Google Scholar]
- Paus T, Barrett J. Transcranial magnetic stimulation of the human frontal cortex: implications for rTMS treatment of depression. J. Psychiatry Neurosci. 2004;29:268–277. [PMC free article] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson C.J, Comeau R, Peters T, Evans A. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J. Neurosci. 1997;17:3178–3184. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Castro-Alamancos M, Petrides M. Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur. J. Neurosci. 2001;14:1405–1411. doi: 10.1046/j.0953-816x.2001.01757.x. [DOI] [PubMed] [Google Scholar]
- Payne B.R, Lomber S.G, Villa A.E, Bullier J. Reversible deactivation of cerebral network components. Trends Neurosci. 1996;19:535–542. doi: 10.1016/s0166-2236(96)10061-8. [DOI] [PubMed] [Google Scholar]
- Penfield W, Rasmussen A.T. Macmillan; New York: 1950. The cerebral cortex of man. [Google Scholar]
- Penny W, Ghahramani Z, Friston K. Bilinear dynamical systems. Phil. Trans. R. Soc. B. 2005;360:983–993. doi: 10.1098/rstb.2005.1642. doi:10.1098/rstb.2005.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth M.F.S, Paus T, Sipila P. The role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J. Neurophysiol. 2002;87:2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- Scoville W.B, Milner B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Burkhalter A. Different balance of excitation and inhibition in forward and feedback circuits of rat visual cortex. J. Neurosci. 1996;16:7353–7365. doi: 10.1523/JNEUROSCI.16-22-07353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Burkhalter A. Role of GABAB receptor-mediated inhibition in reciprocal interareal pathways of rat visual cortex. J. Neurophysiol. 1999;81:1014–1024. doi: 10.1152/jn.1999.81.3.1014. [DOI] [PubMed] [Google Scholar]
- Siebner H.R, Willoch F, Peller M, Auer C, Boecker H, Conrad B, Bartenstein P. Imaging brain activation induced by long trains of repetitive transcranial magnetic stimulation. NeuroReport. 1998;9:943–948. doi: 10.1097/00001756-199803300-00033. [DOI] [PubMed] [Google Scholar]
- Siebner H.R, Peller M, Willoch F, Minoshima S, Boecker H, Auer C, Drzezga A, Conrad B, Bartenstein P. Lasting cortical activation after repetitive TMS of the motor cortex: a glucose metabolic study. Neurology. 2000;54:956–963. doi: 10.1212/wnl.54.4.956. [DOI] [PubMed] [Google Scholar]
- Siebner H.R, Peller M, Bartenstein P, Willoch F, Rossmeir C, Schwaiger M, Conrad B. Activation of frontal premotor areas during suprathreshold transcranial magnetic stimulation of the left primary sensorimotor cortex: a glucose metabolic PET study. Hum. Brain Mapp. 2001;12:157–167. doi: 10.1002/1097-0193(200103)12:3<157::AID-HBM1012>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella A, Paus T. Cerebral blood-flow changes induced by paired-pulse transcranial magnetic stimulation of the primary motor cortex. J. Neurophysiol. 2001;85:2624–2629. doi: 10.1152/jn.2001.85.6.2624. [DOI] [PubMed] [Google Scholar]
- Strafella A, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J. Neurosci. 2001;21:1–4. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella A.P, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126:2609–2615. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- Takada M, Tokuno H, Nambu A, Inase M. Corticostriatal projections from the somatic motor areas of the frontal cortex in the macaque monkey: segregation versus overlap of input zones from the primary motor cortex, the supplementary motor area, and the premotor cortex. Exp. Brain Res. 1998;120:114–128. doi: 10.1007/s002210050384. [DOI] [PubMed] [Google Scholar]
- Vanduffel W, Payne B.R, Lomber S.G, Orban G.A. Functional impact of cerebral connections. Proc. Natl Acad. Sci. USA. 1997;94:7617–7620. doi: 10.1073/pnas.94.14.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn K.J, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motor cortical inhibition induced by blockade of GABA uptake in humans. J. Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Rothwell J.C, Ridding M.C. Interaction between intracortical inhibition and facilitation in human motor cortex. J. Physiol. 1996a;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff B.J, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann. Neurol. 1996b;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]