Abstract
Early endocytic vesicles loaded with Texas Red asialoorosomucoid were prepared from mouse liver. These vesicles bound to microtubules in vitro, and upon ATP addition, they moved bidirectionally, frequently undergoing fission into two daughter vesicles. There was no effect of vanadate (inhibitor of dynein) on motility, whereas 5′-adenylylimido-diphosphate (kinesin inhibitor) was highly inhibitory. Studies with specific antibodies confirmed that dynein was not associated with these vesicles and that Kif5B and the minus-end kinesin Kifc1 mediated their plus- and minus-end motility, respectively. More than 90% of vesicles associated with Kifc1 also contained Kif5B, and inhibition of Kifc1 with antibody resulted in enhancement of plus-end–directed motility. There was reduced vesicle fission when either Kifc1 or Kif5B activity was inhibited by antibody, indicating that the opposing forces resulting from activity of both motors are required for fission to occur. Immunoprecipitation of native Kif5B by FLAG antibody after expression of FLAG-Kifc1 in 293T cells indicates that these two motors can interact with each other. Whether they interact directly or through a complex of potential regulatory proteins will need to be clarified in future studies. However, the present study shows that coordinated activity of these kinesins is essential for motility and processing of early endocytic vesicles.
INTRODUCTION
Receptor-mediated endocytosis is a process in which ligands bind to specific cell surface receptors and internalize via clathrin-coated pits. After internalization the clathrin coat is released and uncoated vesicles mature into early endosomes (Mellman, 1996; Mukherjee et al., 1997; Marsh and McMahon, 1999; Higgins and McMahon, 2002; Perrais and Merrifield, 2005). After acidification of early endosomes, ligands such as asialoorosomucoid (ASOR) that are destined for lysosomes dissociate from their receptors (Harford et al., 1983a,b), and a series of fission events results in their segregation into separate daughter vesicles (Wolkoff et al., 1984; Mellman, 1996; Mukherjee et al., 1997; Murray and Wolkoff, 2003). The resulting ligand-enriched late endosomes traffic to lysosomes for degradation, whereas the receptor-containing daughter endosomes traffic back to the cell surface where receptor is reused (Harford et al., 1983a; Wolkoff et al., 1984; Mukherjee et al., 1997). Previous studies indicated that this segregation event requires an intact microtubule cytoskeleton (Goltz et al., 1992; Novikoff et al., 1996; Murray et al., 2000; Bananis et al., 2003, 2004). In more recent studies, microtubule-based endosome motility and segregation were reconstituted in vitro using fluorescent early endosomes prepared from rat liver 5 min after portal venous injection of Texas Red-labeled ASOR, a substrate for the hepatocyte-specific asialoglycoprotein receptor (ASGPR) (Bananis et al., 2000, 2003, 2004; Murray et al., 2000; Murray and Wolkoff, 2003). On addition of ATP to the in vitro assay, these vesicles moved bidirectionally along microtubules.
This in vitro system has permitted identification of native endogenous proteins that are required for microtubule-based processing of endocytic vesicles. Using these tools, we found that plus-end motility of early endocytic vesicles from rat liver was mediated by the conventional kinesin Kif5B (standardized nomenclature kinesin-1) (Miki et al., 2003, 2005; Lawrence et al., 2004), and minus-end motility was mediated by Kifc2 (standardized nomenclature kinesin-14B) (Bananis et al., 2000, 2003, 2004; Murray et al., 2000). Whereas Kifc2 was initially described as a brain-specific minus-end–directed kinesin (Hanlon et al., 1997; Saito et al., 1997), our studies showed that it was highly associated with these vesicles prepared from rat liver (Bananis et al., 2003). Although the rat is a convenient experimental animal, the power of genetic models that have been established in the mouse led us, in the present study, to examine microtubule-based motility and processing of early endocytic vesicles derived from mouse liver. Although, based on rat studies, we expected to see altered endocytic processing of ASOR by Kifc2 knockout mice (Yang et al., 2001a), we found no differences as compared with wild-type mice. This provided the rationale to define motors that mediate early endocytic vesicle motility in mouse liver and to investigate interactions of these vesicle-associated motors with each other. Such interactions may be of great importance in coordinating activities of opposing motors to regulate endosome fission events as well as trafficking of vesicles to specific destinations within the cell.
MATERIALS AND METHODS
Chemicals and Reagents
ASOR was prepared from human orosomucoid (Sigma-Aldrich, St. Louis, MO) by acid hydrolysis (Stockert et al., 1980) and labeled with Texas Red (Murray et al., 2000) or 125I (Wolkoff et al., 1984) as described previously. Rabbit polyclonal antibody to the 17 N-terminal amino acids of the H1 subunit of the human ASGPR, which also cross-reacts with mouse ASGPR (Treichel et al., 1994), and rabbit polyclonal antibody against Kifc1 (Zhang and Sperry, 2004) were prepared as described previously. Rabbit polyclonal antibody against Kifc3 was purchased from Protein Tech Group (Chicago, IL). Mouse monoclonal immunoglobulin G (IgG) against dynein intermediate chain (IC) was purchased from Chemicon International (Temecula, CA). Tubulin was purchased from Cytoskeleton (Denver, CO). Rabbit antiserum against kinesin-1 (Kif5B) was provided by Dr. Lawrence S.B. Goldstein (University of California, San Diego, La Jolla, CA). Monoclonal antibody against kinesin-1 heavy chain (H2) was generously provided by Dr. Scott Brady (University of Illinois, Chicago, IL). Kinesin light chain (KLC) antibody was purchased from Chemicon International. Cy2- and Cy5-labeled secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA) and Alexa 488-labeled secondary antibody was purchased from Molecular Probes (Eugene, OR). 125Iodine was purchased from GE Healthcare (Little Chalfont, Buckinghamshire, United Kingdom). All other reagents were from Sigma-Aldrich unless otherwise stated.
Animals
Wild-type C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Kifc2 knockout mice were kindly provided by Dr. Lawrence S.B. Goldstein. Male Sprague-Dawley rats (200–250 g) were purchased from Taconic Farms (Germantown, NY). All animal procedures were approved by the Animal Institute Committee of the Albert Einstein College of Medicine (Bronx, NY).
Immunoblot Analysis
Immunoblots were performed as we have described previously (Bananis et al., 2004). In brief, protein samples were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) under reducing conditions (100 mM dithiothreitol [DTT]) and transferred to a polyvinylidene difluoride membrane (PerkinElmer Life and Analytical Sciences, Boston, MA). The membrane was blocked with Tris-buffered saline (TBS) (50 mM Tris-HCl and 150 mM NaCl, pH 7.6) containing 0.1% Tween 20 and 10% nonfat dried milk before incubation with primary antibody diluted appropriately in TBS, 0.1% Tween 20, and 2% nonfat dried milk.
Preparation of FLAG-Kifc1 and FLAG-Kifc2 Expression Plasmids
Kifc1 cDNA (Zhang and Sperry, 2004) was cloned into the pFLAG-CMV-5c vector (Sigma-Aldrich) by using BamHI and HindIII restriction sites. Kifc2 cDNA (kindly provided by Dr. Lawrence S.B. Goldstein) was cloned into the pFLAG-CMV-5a vector (Sigma-Aldrich) by using HindIII and EcoRV restriction sites. Transient transfection of 293T cells with these plasmids was performed using PolyFect transfection reagent (QIAGEN, Valencia, CA) according to the manufacturer's instructions. Cells were harvested 2 d after transfection, washed with phosphate-buffered saline (PBS), and lysates used for further studies as described below.
Preparation of Brain Lysate
Mouse brain was homogenized in radioimmunoprecipitation assay buffer (50 mM Tris-Cl, pH 8.0, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, and 150 mM NaCl) containing 1:50 protease inhibitor (catalog no. P-8340; Sigma-Aldrich). The homogenate was then subjected to centrifugation at 30,000 rpm for 30 min in an SW60 rotor at 4°C. The supernatant was collected and stored at −80°C until use.
Preparation of Antibody to Kifc2
A peptide (GTPSSLSTDTPLTGTSC) containing 17 amino acids near the carboxy terminus (amino acids 746–762) of Kifc2 was synthesized by The Laboratory for Macromolecular Analysis and Proteomics at the Albert Einstein College of Medicine. This peptide was linked to maleimide activated keyhole limpet hemocyanin (Pierce Chemical, Rockford, IL) according to the manufacturer's directions, and this was used for immunization of rabbits by Covance Research Products (Danver, PA). Crude antiserum was purified on SulfoLink gel (Pierce Chemical) to which the peptide was coupled according to the manufacturer's protocol. Specificity of the antibody was assessed by immunoblot of liver endocytic vesicles, brain lysate, and expressed FLAG-Kifc2 (see below) in the presence and absence of peptide.
Isolation and Culture of Mouse Hepatocytes
Hepatocytes were isolated from mice after perfusion of the liver with collagenase type 1 (Worthington Biochemicals, Lakewood, NJ) (Xu et al., 1998). Viability of cells was ∼90% as judged by trypan blue exclusion. For studies of 125I-ASOR processing, 1.5 × 106 cells in Waymouth's 752/1 medium containing 25 mM HEPES, pH 7.2, 5% heat-inactivated fetal bovine serum, 26 mM NaHCO3, 5 μg/ml bovine insulin, 100 IU/ml penicillin, and 0.1 mg of streptomycin were plated in each 60-mm culture dish (Primaria; BD Biosciences, Franklin Lakes, NJ). The medium was changed after 2 h, and the cells were maintained in culture for 16–18 h in a 5% CO2 atmosphere at 37°C (Wolkoff et al., 1984). For immunofluorescence studies, cells were cultured on MatTek culture dishes (MatTek, Ashland, MA) coated with 0.5 mg/ml Matrigel (BD Biosciences) using hepatozyme medium (Invitrogen, Carlsbad, CA).
Studies of 125I-ASOR Internalization and Degradation by Overnight Cultured Mouse Hepatocytes
Surface binding, internalization, and degradation of 125I-ASOR by cultured hepatocytes were assayed as described in previous studies (Wolkoff et al., 1984; Samuelson et al., 1988). In brief, 1 μg/ml 125I-ASOR (6700 cpm/ng) was added to hepatocyte monolayers in ice-cold binding medium (135 mM NaCl, 0.81 mM MgSO4, 1.2 mM MgCl2, 27.8 mM glucose, 2.5 mM CaCl2, and 25 mM HEPES, pH 7.2) and incubated for 60 min at 4°C. Cells were washed four times with ice-cold binding medium to remove unbound ASOR, and then the surface-labeled cells were incubated at 37°C for 0–90 min. Ligand degradation was quantified as radioactivity remaining soluble after addition of an equal volume of 20% trichloroacetic acid, 4% phosphotungstic acid to the incubation medium. Cells were washed twice and surface binding was quantified as radioactivity released following incubation in 20 mM EGTA in 0.15 M NaCl, 0.02 M Tris-Cl, pH 7.6. Nonspecific binding was assessed by inclusion of 100 μg of unlabeled ASOR in the initial incubation with 125I-ASOR.
Immunofluorescence Studies in Overnight Cultured Mouse Hepatocytes
Cultured cells were exposed to 10 μg/ml Texas Red ASOR for 5 min at 37°C in hepatozyme medium, washed twice with warm PBS, and snap-frozen at −80°C with a minimum of PBS to cover the cells. For immunostaining, cells were thawed rapidly and fixed at room temperature for 15 min in 4% formaldehyde at pH 7.4 in 0.25 M sucrose, 5 mM MgCl2, 5 mM EGTA, and 35 mM 1,4-piperazinediethanesulfonic acid (PIPES). They were washed with PBS containing 5 mg/ml casein and incubated for 40 min in Kifc1 antibody diluted 1:100 in this solution. After six washes with PBS containing 5 mg/ml casein, cells were incubated for 40 min in Alexa 488-labeled secondary antibody diluted 1:1000. They were washed extensively and observed by fluorescence microscopy as described below.
Endosome Isolation and In Vitro Motility Assay
Texas Red-labeled early endocytic vesicles were prepared from mouse or rat liver as described previously (Bananis et al., 2000, 2003; Murray et al., 2000). In brief, livers were harvested 5 min after portal venous injection of 50 μg of Texas Red-labeled ASOR. A postnuclear supernatant was prepared after Dounce homogenization of the liver and subjected to chromatography on a Sephacryl S200 (GE Healthcare) column. Vesicle-enriched fractions were pooled and centrifuged at 200,000 × g for 135 min on a sucrose step gradient consisting of 1.4, 1.2, and 0.25 M sucrose in a Beckman SW60 rotor. Vesicles were collected from the 1.2 M/0.25 M sucrose interface and stored at −80°C until used. Motility assays were performed in a 3-μl chamber consisting of two pieces of double-sided tape sandwiched between optical glass as described previously (Murray et al., 2002). The chamber was coated with 0.03 mg/ml DEAE-dextran (GE Healthcare), and rhodamine-labeled, Taxol-stabilized microtubules were added and incubated for 3 min at room temperature (Bananis et al., 2000, 2003, 2004). The chamber was washed three times with PMEE motility buffer (35 mM PIPES-K2, 5 mM MgCl2, 1 mM EGTA, 0.5 mM EDTA, 4 mM DTT, 20 μM Taxol, and 2 mg/ml BSA) containing 5 mg/ml casein followed by three washes with PMEE motility buffer without casein. Vesicles were then flowed into the chamber, incubated for 10 min to permit binding to microtubules, and washed with PMEE motility buffer containing 10 mM ascorbic acid. Motility was initiated by the addition of 50 μM ATP without a regenerating system in the presence or absence of 5 μM vanadate or 1 mM 5′-adenylylimido-diphosphate (AMP-PNP). In some studies to quantify directional motility, polarity-marked, rhodamine-labeled microtubules were used. These polarity marked microtubules were prepared by first polymerizing seeds containing 10 mg/ml tubulin (1:75 labeled/unlabeled) in polymerizing buffer (80 mM PIPES, 1 mM EGTA, 1 mM MgCl2, 1 mM GTP, and 3% glycerol, pH 6.8) at 37°C for 5 min. The seeds were sheared by pipetting up and down and subjected to extension at their ends after addition of 2.5 mg/ml tubulin (1:6 labeled/unlabeled) for 6 min. The reaction was stopped by adding polymerizing buffer containing 20 μM Taxol. Microtubules were pelleted by centrifugation for 4 min at 15 psi at room temperature in a Beckman airfuge and resuspended in polymerizing buffer containing 20 μM Taxol. In some experiments microtubule-bound vesicles were incubated with antibodies against specific motor proteins for 6 min before the addition of ATP (Bananis et al., 2003). In some immunofluorescence colocalization studies, chambers were not coated with microtubules, and vesicles were bound directly to the glass surface as we described in previous studies (Bananis et al., 2000).
Immunofluorescence Studies of Vesicles In Vitro
Glass- or microtubule-bound endocytic vesicles were incubated with appropriately diluted primary antibodies in PMEE buffer (35 mM PIPES-K2, 5 mM MgCl2, 1 mM EGTA, 0.5 mM EDTA, 20 μM Taxol, and 2 mg/ml BSA) containing 5 mg/ml casein for 6 min at room temperature, blocked with motility buffer containing 5 mg/ml casein, and incubated with fluorescently labeled affinity-purified secondary antibody for 5 min. For simultaneous immunolocalization of two proteins, vesicles were first washed with motility buffer containing 5 mg/ml casein after sequential incubation with the first set of primary and fluorescent secondary antibodies. This was followed by incubations with the second set of primary and contrasting fluorescent secondary antibodies. The chambers were washed with PMEE motility buffer containing 10 mM ascorbic acid and examined by immunofluorescence microscopy (Bananis et al., 2000, 2003, 2004).
Image Analysis
Imaging was performed with a 60× 1.4 numerical aperture Olympus objective on an Olympus 1 × 71 inverted microscope maintained either at 28°C for mouse vesicle studies or at 37°C for rat vesicle studies and containing automated excitation and emission filter wheels. Data were collected through a CoolSNAP HQ cooled charge-coupled device (Photometrics, Roper Scientific, Tucson, AZ) camera regulated by MetaMorph (Molecular Devices, Sunnyvale, CA) software. Fluorescent images were analyzed using ImageJ (National Institutes of Health public domain; http://rsb.info.nih.gov/ij/) and Adobe Photoshop version 6.0 (Adobe Systems, San Jose, CA), and colocalizations were scored manually. Colocalization of ASOR-containing vesicles with antibodies to candidate proteins was quantified by first determining the number of fluorescent vesicles in the rhodamine (ASOR) channel and then overlaying the green channel to find the number of colocalized vesicles. For motility studies, time-lapse movies were taken at 1 frame per second for 60 s. Movies were analyzed using ImageJ software.
Interaction of Kif5B and FLAG-Kifc1
Preliminary studies showed that 293T cells have endogenous expression of Kif5B (kinesin-1). 293T cells transiently transfected with pFLAG-Kifc1 or pFLAG vector alone, as described above, were incubated for 60 min on ice in immunoprecipitation buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 50 mM KCl, 10 mM EDTA, 10 mM EGTA, 1.5% Triton X100, and 0.75% NP-40) containing protease inhibitors (catalog no. P-8340; Sigma-Aldrich). The lysate was centrifuged at 14,000 rpm for 10 min at 4°C, and the supernatant was incubated with nonimmune IgG-linked agarose (Sigma-Aldrich) for 3 h followed by overnight incubation with anti-FLAG M2-linked agarose (Sigma-Aldrich) according to the manufacturer's instructions. Immunoabsorbed protein was identified by immunoblot. In companion studies, 293T cells were cotransfected with FLAG-Kifc1 and a pcDNA3 expression plasmid encoding KLC2 (Ligon et al., 2004), kindly provided by Dr. Erika Holzbaur (University of Pennsylvania). These cotransfected cells were processed as described above to assess interaction of Kifc1 with KLC2.
Statistical Analysis
Statistical analysis was performed using chi-square or Student's t test as appropriate.
RESULTS
Binding, Internalization, and Degradation of 125I-ASOR by Wild-Type and Kifc2 Knockout Mouse Hepatocytes
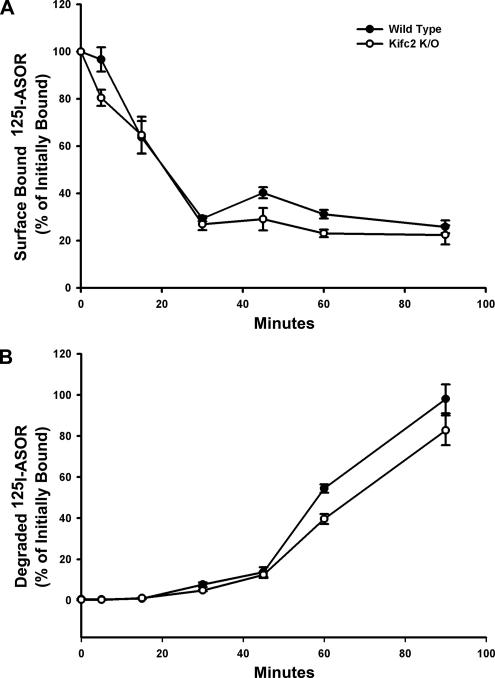
Based upon previous studies in rat early endocytic vesicles showing that Kifc2 mediated their minus-end–directed motility on microtubules (Bananis et al., 2003), we hypothesized that Kifc2 knockout mice would show a defect in endocytic processing of ASOR. This was examined by quantifying single-wave processing of 125I-ASOR by overnight cultures of hepatocytes isolated from livers of wild-type and knockout mice. In cells from both types of mouse, internalization of cell surface bound 125I-ASOR was rapid with ∼70% internalized within 30 min of incubation at 37°C (Figure 1A). Degradation of internalized ligand was nearly complete by 90 min in both wild-type and knockout cells (Figure 1B), indicating that internalization and processing of 125I-ASOR in mouse liver do not require Kifc2, but likely use another microtubule-based motor(s). This was examined in subsequent studies in vitro as described below.
Figure 1.
125I-ASOR binding and degradation in wild-type and Kifc2 knockout mouse hepatocytes. (A) Binding of 125I-ASOR to the surface of hepatocytes was assayed as described in Materials and Methods. The percentage of initially bound 125I-ASOR remaining on the surface of replicate plates at varied times after incubation at 37°C in hepatocytes from wild-type (closed circles) and Kifc2 knockout (open circles) mice is shown. (B) Release of degradation products of 125I-ASOR into the medium was assayed as described in Materials and Methods and is shown as percentage of initially bound 125I-ASOR. Each study was done in triplicate, and the error bar represents SEM.
Immunoblot Detection of Kifc2 in Mouse Liver
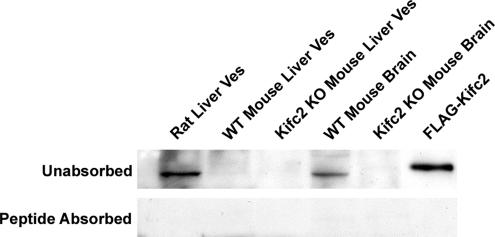
We showed previously that early endocytic vesicles prepared from rat liver were associated with the minus-end kinesin Kifc2 and the plus-end kinesin Kif5B (Bananis et al., 2003). The presence of Kifc2 in vesicles prepared from mouse liver was assayed by immunoblot. As seen in Figure 2, Kifc2 was detectable in rat liver vesicles and wild-type mouse brain lysate but not in wild-type mouse liver vesicles. As expected, it was not detectable in liver or brain from Kifc2 knockout mice. Immunoreactivity was removed by preabsorption of antiserum with the peptide to which it was generated (Figure 2, bottom). These results indicate that, as distinct from the rat, mouse liver early endocytic vesicles are not associated with Kifc2.
Figure 2.
Immunoblot analysis of Kifc2. Immunoblot was performed after 7.5% SDS-PAGE of liver vesicles or brain lysate from wild-type or Kifc2 knockout mice as indicated (15 μg protein/lane). Rat liver early endocytic vesicles and a lysate prepared from 293T cells overexpressing FLAG-Kifc2 were used as positive controls. Antiserum was used at 1:1000 dilution before (top) or after (bottom) absorption for 3 h at room temperature with 10 μg/ml peptide against which it was made.
Preparation of Early Endocytic Vesicles from Mouse Liver
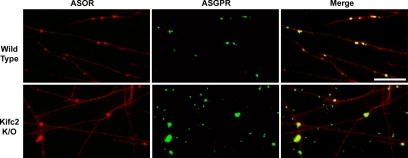
To identify microtubule-based motors that are required for motility, fluorescent early endocytic vesicles were prepared from mouse liver 5 min after portal venous injection of 50 μg of Texas Red ASOR. The procedure was identical to that used for rat liver (Bananis et al., 2000, 2003; Murray et al., 2000; Murray and Wolkoff, 2005). Vesicles were attached to a glass motility chamber, and ASGPR was visualized by immunofluorescence. Its distribution on vesicles was compared with that of Texas Red ASOR. As illustrated in Figure 3, 95% of ASOR-containing vesicles isolated from both wild-type and Kifc2 knockout mice were associated with the ASGPR, indicating that these vesicles represent a population of early (presegregation) endocytic vesicles similar to the early endocytic vesicles that we described previously from rat liver (Bananis et al., 2000).
Figure 3.
Colocalization of ASOR and its receptor in mouse liver early endocytic vesicles in vitro. Texas Red-labeled ASOR containing wild-type (top) and Kifc2 knockout (bottom) mouse liver early endocytic vesicles were bound to rhodamine-labeled, Taxol-stabilized microtubules and visualized in the rhodamine channel (left). Microtubule bound vesicles were incubated with antibody against the ASGPR followed by Cy2-labeled secondary antibody and visualized in the fluorescein isothiocyanate (FITC) channel (middle). Yellow vesicles in the merged figure (right) indicate colocalization of ligand (ASOR) and receptor (ASGPR). Bar, 10 μm.
Microtubule-based Motility and Fission of Mouse Liver Early Endocytic Vesicles
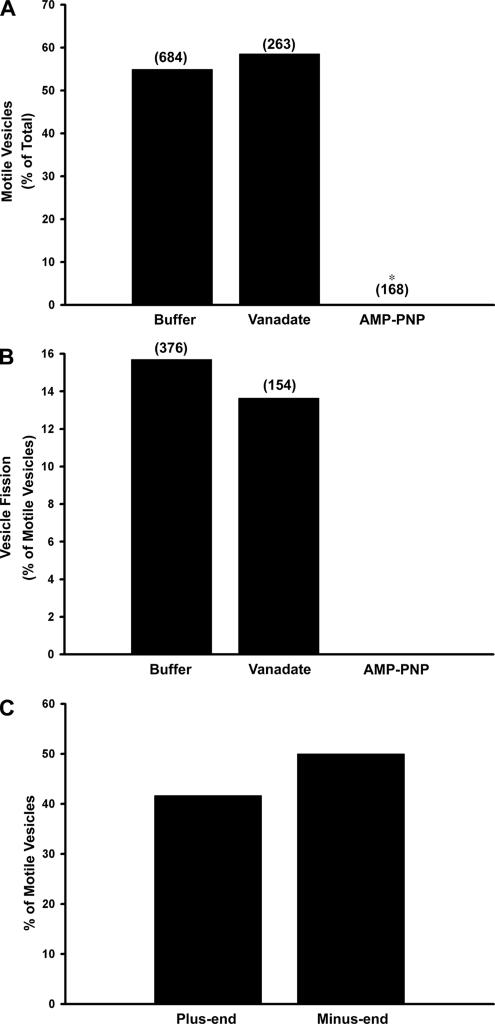
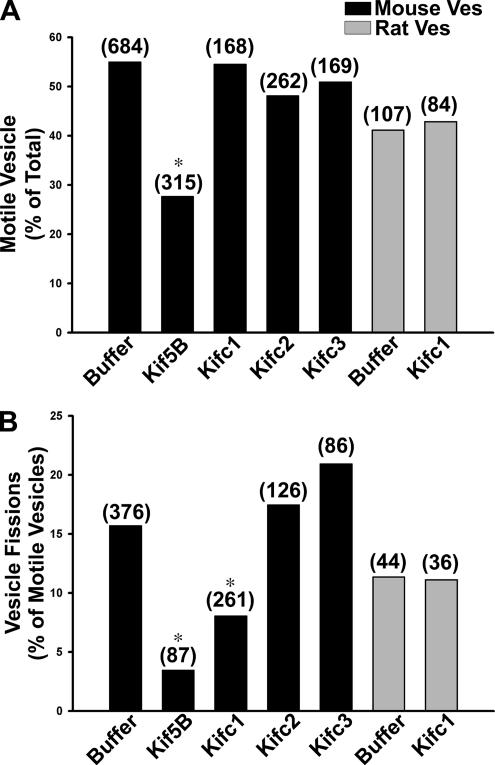
These early endocytic vesicles from mouse liver were subjected to time-lapse fluorescent microscopy to quantify their interaction with and motility along microtubules. In contrast to previous studies using either rat endocytic vesicles (Bananis et al., 2000) or vesicles containing herpes simplex virus (Lee et al., 2006), when mouse early endocytic vesicles were assayed at 37°C, there was so much motility that microtubules that should have been fixed to the slide began gliding, making the analysis very difficult (data not shown). We found that this problem of microtubule movement and detachment was markedly reduced when motility assays were conducted on mouse vesicles at 28°C rather than at 37°C, and subsequent studies were performed at this lower temperature. On addition of 50 μM ATP, 55% of the microtubule-bound wild-type mouse fluorescent early endocytic vesicles were motile (Figure 4A and Supplemental Movie 1) with an average nonzero velocity of 0.60 ± 0.04 μm/s (n = 52, mean ± SEM). Among these motile vesicles 16% underwent fission (Figure 4B and Supplemental Movie 1). When the vesicles were preincubated with 5 μM vanadate, an inhibitor of the minus-end–directed motor dynein (Kobayashi et al., 1978; Bananis et al., 2000, 2004; Murray et al., 2000), motility and fission were essentially unchanged (Figure 4, A and B). However, pretreatment with 1 mM AMP-PNP, a kinesin inhibitor (Vale et al., 1992; Bananis et al., 2000, 2004; Murray et al., 2000), before ATP addition inhibited vesicle motility and fission completely (Figure 4, A and B). These results suggest that microtubule-based motility of mouse early endocytic vesicles is mediated by kinesins and not dynein, similar to results obtained with rat early endocytic vesicles (Bananis et al., 2000). Early endocytic vesicles prepared from Kifc2 knockout mouse liver behaved identically (data not shown).
Figure 4.
Effect of vanadate and AMP-PNP on motility, fission, and directional motility of early endocytic vesicles from wild-type mouse liver. Fluorescent early endocytic vesicles were bound to microtubules. (A) Motility was initiated by addition of 50 μM ATP in the absence or presence of 5 μM vanadate or 1 mM AMP-PNP. The total number of vesicles counted is indicated above the bars. *p < 0.0005 compared with control, and values represent data compiled from 10 (buffer) and four (vanadate and AMP-PNP) independent experiments. (B) The fraction of motile vesicles undergoing fission is shown. The total number of motile vesicles examined is indicated above the bars. (C) Polarity marked microtubules were prepared and bound to the glass surface of the optical chamber using DEAE dextran as described in Materials and Methods. Early endocytic vesicles were then perfused into the chamber. The bars indicate the percentage of motile vesicles moving toward the plus- or minus-ends of the microtubules after addition of 50 μM ATP. Motility of 36 vesicles from five independent experiments was examined in this representative study.
Assessment of Directional Motility
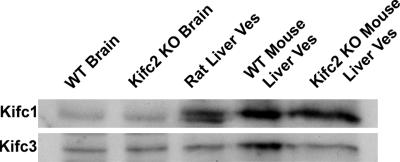
The preceding studies examined total vesicle motility, but they did not provide information regarding the direction that vesicles moved on microtubules. To answer this question, vesicle motility was assayed on directionally marked microtubules. These studies showed that 50% of the motile wild-type early endocytic vesicles moved toward the minus-end, whereas 42% moved toward the plus-end of microtubules (Figure 4C). The remainder of the vesicles (8%) moved bidirectionally, indicating activity of both plus- and minus-end motors on these vesicles. Early endocytic vesicles prepared from Kifc2 knockout mouse liver behaved identically (Supplemental Movie 2). These data show that early endocytic vesicles prepared from wild-type or Kifc2 knockout mouse liver move bidirectionally on microtubules and that this movement is driven by plus- and minus-end–directed kinesins. Because the vesicles from the knockout mice are devoid of Kifc2, the kinesin that mediates minus-end motility of rat early endocytic vesicles, we investigated whether another minus-end kinesin was associated with these vesicles. Aside from Kifc2, only four other minus-end kinesins, Kifc1, -3, -4, and -5, have been described in mammals (Yang et al., 1997, 2001b, 2006; Yang and Goldstein, 1998; Navolanic and Sperry, 2000; Miki et al., 2001, 2003; Noda et al., 2001; Xu et al., 2002; Yang and Sperry, 2003; Zhang and Sperry, 2004). Kifc1 and Kifc3 were found to be associated with membrane-bound intracellular organelles (Yang and Goldstein, 1998; Hoang et al., 1999; Noda et al., 2001; Xu et al., 2002; Yang and Sperry, 2003; Zhang and Sperry, 2004; Yang et al., 2006), whereas Kifc4 and Kifc5 are primarily active in mitosis (Yang et al., 1997; Zhang and Sperry, 2004). As seen in Figure 5, immunoblot analysis showed that Kifc1 and Kifc3 were both present in liver and brain of wild-type and Kifc2 knockout mice. Further studies were then performed to determine whether either of these motors was functionally associated with early endocytic vesicles.
Figure 5.
Immunoblot analyses of Kifc1 and Kifc3. Immunoblots were performed after 10% SDS-PAGE of wild-type mouse and Kifc2 knockout mouse brain lysates, rat liver early endocytic vesicles, and wild-type or Kifc2 knockout mouse liver early endocytic vesicles (25 μg protein/lane) by using Kifc1 (top) or Kifc3 (bottom) antibodies.
Immunofluorescence Colocalization of Motor Molecules with Early Endocytic Vesicles
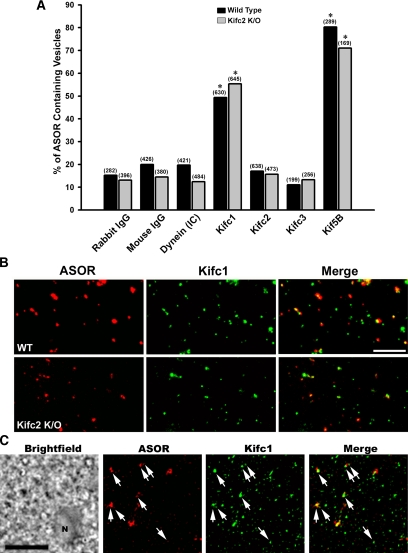
Using specific antibodies, we quantified the association of the candidate minus-end–directed kinesins Kifc1 and Kifc3 with Texas Red ASOR-loaded early endocytic vesicles prepared from wild-type and Kifc2 knockout mice. Kifc2 antibody was used as a control, because it did not detect the protein on Western blot of mouse vesicle preparations. Corresponding studies were also performed using antibodies to dynein and the plus-end–directed kinesin Kif5B. As seen in Figure 6A, immunofluorescence microscopy analysis showed that only antibodies to Kifc1 and Kif5B colocalized with these vesicles. The relatively low degree of colocalization of antibodies to dynein, Kifc2, and Kifc3 was similar to that seen with nonimmune IgG (Figure 6A). Representative immunofluorescence images showing colocalization of Kifc1 with wild-type and Kifc2 knockout mouse early endocytic vesicles are seen in Figure 6B. To confirm that Kifc1 is associated with endocytic vesicles in vivo, overnight cultured mouse hepatocytes were incubated for 5 min at 37°C with 10 μg/ml Texas Red ASOR to label early endocytic vesicles. Cells were then permeabilized by freeze-thaw, fixed, and incubated with antibody to Kifc1 and Alexa 488-labeled secondary antibody. As seen in Figure 6C, most of the ASOR-containing vesicles in these cells were associated with Kifc1.
Figure 6.
Immunofluorescence colocalization of motors with endocytic vesicles. (A) Texas Red ASOR-containing early endocytic vesicles from wild-type or Kifc2 knockout mouse livers were perfused into optical chambers and incubated with nonimmune IgG, or dynein, Kifc1, Kifc2, Kifc3, or Kif5B antibodies followed by incubation with Cy2-labeled secondary antibody. Vesicles were visualized in the rhodamine channel, and antibody staining was visualized in the FITC channel. The percentage of ASOR-containing vesicles colocalizing with each antibody is shown. The total number of vesicles examined is indicated above the bars. *p < 0.0005 compared with nonimmune IgG. (B) Representative fluorescence micrographs in which colocalization of ASOR-containing vesicles (red) with Kifc1 (green) was examined. The yellow vesicles in the merged images (right column) indicate colocalization. (C) Overnight cultured mouse hepatocytes were incubated with 10 μg/ml Texas Red ASOR for 5 min at 37°C. They were then snap-frozen at −80°C and processed for immunodetection of Kifc1 as described in Materials and Methods. A bright-field image showing a portion of a hepatocyte (N, nucleus) is in the panel on the left, and vesicles in the same field containing ASOR or Kifc1 are in the next two panels as indicated. A merged fluorescence image is on the right, and arrows indicate ASOR-containing vesicles that also contain Kifc1. Bar, 10 μm.
Effects of Motor Antibodies on Mouse Liver Early Endocytic Vesicle Motility and Fission
To assess which vesicle-associated motors mediated microtubule-based motility, we quantified motility and fission in the presence or absence of specific motor antibodies. Using this strategy in previous studies, we showed that antibodies to plus-end–directed conventional kinesin Kif5B and minus-end–directed kinesin Kifc2 inhibited motility and fission of early endocytic vesicles prepared from rat liver (Bananis et al., 2003, 2004). In the present study, preincubation of wild-type mouse liver early endocytic vesicles with antibody to Kif5B reduced vesicle motility by ∼50% (Figure 7A). Although antibody against Kifc1 did not alter the number of motile vesicles from either mouse or rat (Figure 7A), the number of fission events was significantly reduced by ∼50% in the mouse (Figure 7B). There was no effect of this antibody on fission of rat vesicles. Antibodies to Kifc2 or Kifc3 had no effect on motility or fission of mouse early endocytic vesicles (Figure 7). Experiments were also performed to determine whether Kifc1, in addition to Kifc2, is associated with rat early endocytic vesicles. Although Kifc1 was present in the rat liver vesicle preparation (Figure 5), we found little (18%) colocalization of rat early endocytic vesicles with this motor by immunofluorescence assay. These results are in contrast to the >60% colocalization of these vesicles with Kifc2 that we reported previously (Bananis et al., 2003, 2004). Consistent with these findings, Kifc1 antibody did not have any effect on motility or fission of rat early endocytic vesicles (Figure 7).
Figure 7.
Effect of motor antibodies on early endocytic vesicle motility and fission. Fluorescent ASOR-containing endocytic vesicles from wild-type mouse (black) or rat (gray) liver were bound to microtubules and incubated for 6 min in the absence or presence of motor antibodies, as described in Materials and Methods, before addition of 50 μM ATP. The percentage of vesicles moving along microtubules (A) and the percentage of motile vesicles undergoing fission (B) are shown. The total number of vesicles examined in each condition is shown in parentheses and represents data obtained from three to 10 independent experiments. *p < 0.05 compared with buffer control by chi-square analysis.
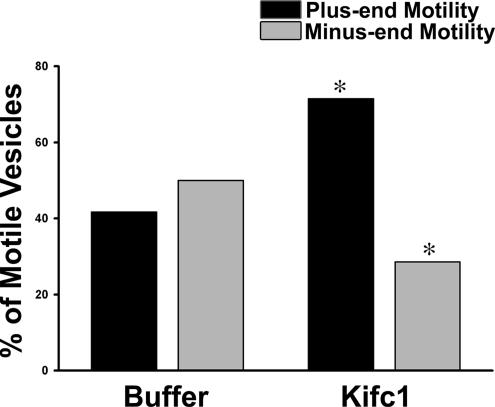
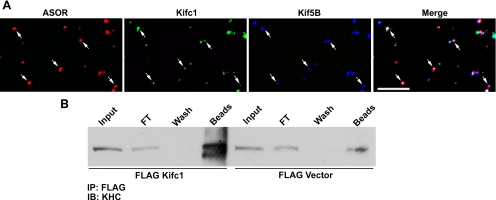
To examine the mechanism by which antibody to Kifc1 inhibited fission but not the number of motile mouse liver early endocytic vesicles, we determined its effect on directional motility. As seen in Figure 8, when early endocytic vesicles from wild-type mouse liver were incubated with Kifc1 antibody, the proportion of vesicles moving toward the minus-end of directionally labeled microtubules fell from 50 to 29%. There was a compensatory increase (from 42 to 71%) of plus-end movement, accounting for the fact that overall motility of the vesicles was unchanged. These results suggest that Kifc1 and Kif5B may coexist on a population of early endocytic vesicles where they act antagonistically. To measure this directly, the colocalization of Kifc1 and Kif5B on ASOR-containing mouse vesicles was analyzed by quantitative immunofluorescence. Two analyses of the image data obtained from each microscopy chamber were performed: one analysis to measure the amount of overlap of Kif5B in the set of vesicles containing Kifc1 and ASOR, and the other analysis to measure the amount of overlap of Kifc1 in the set of vesicles containing Kif5B and ASOR. As seen in Table 1 and the representative immunofluorescence colocalization study in Figure 9A, >90% of the ASOR-containing vesicles that were associated with Kifc1 were also associated with Kif5B. Conversely, ∼68% of the ASOR-containing vesicles that were associated with Kifc1 were also associated with Kif5B (Table 1). These data indicate that inhibition of the minus end kinesin Kifc1 results in unopposed Kif5B activity with consequent augmentation of plus-end movement of these vesicles.
Figure 8.
Effect of Kifc1 antibody on directional motility of mouse liver early endocytic vesicles. Fluorescent ASOR-containing endocytic vesicles from wild-type mouse liver were bound to polarity marked microtubules in an optical chamber. Vesicles were incubated for 6 min in the absence or presence of Kifc1 antibody. The bars indicate the percentage of motile vesicles moving toward the plus- (black) or minus (gray)-ends of microtubules after addition of 50 μM ATP. Motility of 36 vesicles was examined in buffer and 42 in the presence of Kifc1 antibody over five independent experiments for each condition. *p < 0.05 compared with control by chi-square analysis.
Table 1.
Simultaneous association of Kifc1 and Kif5B with early endocytic vesicles
| Analysis 1 | ||
| Group 1 | No. of ASOR-positive vesicles | 301 |
| Group 2 | No. in group 1 positive for Kifc1 | 176 |
| Group 3 | No. in group 2 positive for Kif5B | 162 |
| % of Kifc1 vesicles with Kif5B | 92% | |
| % of ASOR vesicles with KifC1 and Kif5B | 54% | |
| Analysis 2 | ||
| Group 4 | No. of ASOR-positive vesicles | 309 |
| Group 5 | No. in group 4 positive for Kif5B | 235 |
| Group 6 | No. in group 5 positive for Kifc1 | 161 |
| % of Kif5B vesicles with Kifc1 | 69% | |
| % of ASOR vesicles with KifC1 and Kif5B | 52% | |
Texas Red ASOR-containing early endocytic vesicles from wild-type mouse liver were bound to an optical chamber and immunostained for Kifc1 and Kif5B sequentially as described in Materials and Methods. In the first image analysis (analysis 1), ASOR-containing vesicles were identified (group 1), and those that also contained Kifc1 (group 2) were quantified. Next, the fraction of the group 2 vesicles that also contained Kif5B was quantified. Image analysis of ASOR-containing vesicles was also done in the reverse order (analysis 2).
Figure 9.
Interaction of Kifc1 and Kif5B in early endocytic vesicles. (A) Texas Red ASOR-containing early endocytic vesicles from wild-type mouse liver were perfused into optical chambers and incubated with Kifc1 antibody followed by incubation with Alexa 488-labeled secondary antibody. After washing, the vesicles were incubated with antibody to Kif5B followed by Cy5-labeled secondary antibody, as described in Materials and Methods. Vesicles were visualized in the rhodamine channel, and antibody staining was visualized in the FITC (Kifc1) and Cy5 (Kif5B) channels. A typical experiment in which ASOR (red), Kifc1 (green), and Kif5B (blue) were examined is shown. Vesicles in white in the merged image (right) represent early endocytic vesicles that are associated with both motors simultaneously. Bar, 10 μm. (B) To examine whether Kifc1 and Kif5B can exist in a complex, 293T cells that endogenously express Kif5B were transfected with FLAG-Kifc1 or FLAG vector alone, and a FLAG immunoprecipitate was examined by immunoblot by using antibody to the kinesin heavy chain (KHC). Lanes in the immunoblot are cell lysate (input), representing 2% of total volume; material flowing through the anti-FLAG-agarose beads (FT), representing 2% of total volume; material in the buffer wash (wash), representing 2% of total volume; and material released from the beads after incubation in sample buffer (beads), representing 50% of bound material.
Interaction of Kifc1 with Kif5B
Finding that Kifc1 and Kif5B are associated with the same early endocytic vesicles raises the question as to whether they interact with each other. Recent studies showed interaction of Kif5B (kinesin-1) with dynein (Ligon et al., 2004). To examine whether Kifc1 interacts with Kif5B, 293T cells that express Kif5B endogenously (Figure 9B) were transfected with either FLAG-Kifc1 or FLAG vector. Absorption with anti-FLAG agarose beads revealed an abundance of Kif5B in cells transfected with FLAG-Kifc1, but only a small amount under control conditions with FLAG vector alone (Figure 9B). Corresponding kinesin light chain was also found in the immunoabsorbate when reprobed with anti KLC antibody (data not shown). Previous studies suggested that Kif5B interacted through its light chains with the IC of dynein (Ligon et al., 2004). Mouse liver expresses KLC2 (Rahman et al., 1998). To test whether Kifc1 binds to Kif5B through its light chain, 293T cells were cotransfected with expression plasmids encoding FLAG-Kifc1 and KLC2. Both proteins were expressed, but there was no detectable KLC2 in the FLAG immunoabsorbate, suggesting that these proteins do not interact (data not shown). These results support the notion that interaction of Kifc1 and Kif5B requires the Kif5B heavy chain, although we do not as yet know whether these proteins interact directly or via a scaffold of other potentially regulatory proteins.
DISCUSSION
Receptor-mediated endocytosis is a biologically essential process in which ligands, including hormones, toxins, and viruses, interact with specific cell surface receptors and traffic through a series of intracellular vesicular structures along microtubules (Mellman, 1996; Mukherjee et al., 1997; Murray and Wolkoff, 2003). The mechanisms coordinating and regulating this complex process have been the subject of investigation by many laboratories (Bananis et al., 2003, 2004; Maxfield and McGraw, 2004; Macia et al., 2006). In recent studies, we used the hepatocyte-specific asialoglycoprotein receptor system to produce endocytic vesicles from rat liver that were loaded with fluorescent ligand (ASOR), and a microscopy assay to quantify motility and processing of these vesicles on microtubules in vitro was established (Murray and Wolkoff, 2005). These studies showed that early endocytic vesicles prepared from rat liver move bidirectionally on microtubules, by using the plus- and minus-end–directed kinesins Kif5B and Kifc2, respectively (Bananis et al., 2003). Based on these results, we predicted that disruption of Kifc2 function would reduce endocytic processing of ASOR, and availability of a Kifc2 knockout mouse provided the opportunity to test this hypothesis. This mouse model was reported as having normal embryonic viability and no grossly discernible phenotype (Yang et al., 2001a). In conformity with these results, we found that overnight cultured hepatocytes prepared from these mice had normal processing of 125I-ASOR and that Kifc2 was not present in wild-type mouse liver (Figure 2).
These results suggested that in contrast to the rat, Kifc2 plays no role in endocytic vesicle processing in mouse liver. Previous methods for preparation and study of in vitro motility of rat liver early endocytic vesicles were adapted in the present study to mouse liver. As in the rat, Texas Red-containing vesicles prepared from mice were highly associated (95%) with the asialoglycoprotein receptor (Figure 3), indicating that they represent a presegregation population of early endocytic vesicles (Bananis et al., 2000). These vesicles bound to microtubules in vitro and moved bidirectionally after ATP addition. Motility of early endocytic vesicles prepared from Kifc2 knockout mice was indistinguishable from that of wild-type mice. There was no evidence that the minus-end motility of these vesicles was due to substitution of dynein for Kifc2. Specifically, there was no inhibition of vesicle motility in the presence of 5 μM vanadate (Figure 4), a treatment that inhibits dynein-mediated motility (Bananis et al., 2000; Sarkar et al., 2006), and there was no immunocolocalization of dynein with fluorescent ASOR-containing vesicles (Figure 6A). Rather, as was found in studies of early endocytic vesicles from the rat, all motility in both wild-type and Kifc2 knockout mouse vesicles was inhibited by 1 mM AMP-PNP, an inhibitor of kinesin based motility (Vale et al., 1992; Bananis et al., 2000; Murray et al., 2000). A recent study in which HeLa cells were loaded with fluorescent epidermal growth factor for 2–3 min and followed for as long as 30 min was interpreted as showing a role for dynein in processing of early endocytic vesicles (Driskell et al., 2007), in contrast to other studies (Nielsen et al., 1999; Bananis et al., 2003. 2004). As processing of endocytic vesicles is dynamic (Bananis et al., 2004; Sarkar et al., 2006), it is possible that these vesicles had already passed a transition point toward late vesicles, which we have shown are associated with dynein (Bananis et al., 2004). Alternatively, they could represent a different population of early/recycling endocytic vesicles such as those that contain the bile acid transporter sodium taurocholate cotransporting polypeptide (ntcp) (Sarkar et al., 2006). These ntcp-containing vesicles cycle to and from the basolateral plasma membrane of hepatocytes, by using Kif5B and dynein (Sarkar et al., 2006).
Because these data indicated the presence of a minus-end–directed kinesin on mouse early endocytic vesicles and Kifc2 was absent, we considered the presence of other minus-end kinesins, of which only four others have been described previously (Yang et al., 1997, 2001a,b, 2006; Navolanic and Sperry, 2000; Noda et al., 2001; Miki et al., 2003; Yang and Sperry, 2003; Zhang and Sperry, 2004). Within this group, Kifc1 and Kifc3 were associated with membranous organelles (Yang et al., 1997; Navolanic and Sperry, 2000; Noda et al., 2001; Yang et al., 2001b, 2006; Yang and Sperry, 2003; Zhang and Sperry, 2004), whereas Kifc4 and Kifc5 were seen in mitosis where they play a role in chromosome movement (Yang et al., 1997; Zhang and Sperry, 2004). Consequently, we looked for the presence of Kifc1 and Kifc3 on mouse early endocytic vesicles. Immunoblot analysis (Figure 5) showed the presence of both Kifc1 and Kifc3 in mouse vesicle preparations from wild-type and Kifc2 knockout mice. These preparations consist of a mixture of vesicles containing endocytosed fluorescent ligand and other unlabeled vesicles. We found that only Kifc1, but not Kifc3, was substantially associated with the ASOR-containing vesicles as revealed by immunofluorescence colocalization (Figure 6). Association of Kifc3 with ASOR-containing vesicles was at background levels, although it was present on unidentified vesicles that did not contain Texas Red ASOR (data not shown). Approximately 80% of mouse early endocytic vesicles were also associated with the plus-end kinesin Kif5B, similar to results in rat vesicles (Bananis et al., 2000, 2003, 2004).
The findings that >50% of mouse early endocytic vesicles are associated with Kifc1 and >80% with Kif5B suggest that there is a population of vesicles that must be associated simultaneously with both motors. As seen in Table 1, >90% of vesicles that were associated with Kifc1 were also associated with Kif5B. Of the total population of vesicles containing ASOR, approximately half were associated simultaneously with both motors. This is in agreement with two observations. First, that some vesicles moving in one direction along a microtubule stop and then move in the other direction. Second, that plus end motility is increased when minus-end motility is inhibited by incubation of vesicles with antibody to Kifc1 (Figure 8). These data are consistent with the possibility that these motors may be part of a yet to be elucidated protein complex that mediates their coordinate regulation, as has been suggested for vesicle-associated dynein and plus-end kinesins (Brady et al., 1990; Stenoien and Brady, 1997; Waterman-Storer et al., 1997; Ligon et al., 2004). This view is supported in the present study by the finding that Kifc1 and Kif5B can interact with each other as shown by immunoabsorption of native Kif5B by FLAG-Kifc1 expressed in 293T cells (Figure 9B). Whether these motors interact directly or through a complex of potential regulatory proteins will need to be clarified in future studies. It is of interest that in contrast to results with antibody to Kifc1, overall motility of vesicles is reduced after incubation with antibody to Kif5B. This is in agreement with results observed in several previous studies (Brady et al., 1990; Martin et al., 1999; Ligon et al., 2004; Theiss et al., 2005; Sarkar et al., 2006), suggesting that Kif5B activity is dominant over other motor activities (Sarkar et al., 2006). The finding that fission of vesicles is reduced when either Kifc1 or Kif5B activity is inhibited by antibody also suggests that the opposing forces resulting from activity of both motors are required for fission to occur.
Although Kifc1 and Kifc2 are both present in rat liver vesicle preparations (Figure 5), our data indicate that only Kifc2 is used for motility of ASOR-containing rat early endocytic vesicles (Figure 7; Bananis et al., 2003). Thus minus-end–directed motility of early endocytic vesicles is mediated by Kifc2 in the rat and Kifc1 in the mouse. These are genetically distinct proteins with little homology (Saito et al., 1997). Specific regions of a number of kinesins that interact with and bind to cargo have been identified previously (Hirokawa, 1998; Verhey et al., 2001; Smith et al., 2006). In particular, previous studies of Kifc1 identified a 19-amino acid sequence that is required for binding to membrane-bounded organelles (Zhang and Sperry, 2004). This Kifc1-specific sequence has no corresponding region in Kifc2, which presumably has its own unique, although not yet characterized, organelle-interacting sequence. The endocytic vesicle-associated proteins that interact with these binding regions on kinesins are not known, but differences in the protein constituents of rat compared with mouse early endocytic vesicles are likely to be a primary factor in the species difference in minus-end–directed kinesin recruitment.
This study showed that coordinated activity of plus- and minus-end–directed kinesins is essential for motility and processing early endocytic vesicles. Little is known about the role of minus-end kinesins in vesicle trafficking, although their importance in mitosis is clear (Zhang and Sperry, 2004; Goshima et al., 2005; Christodoulou et al., 2006). There are homologues of Kifc1 and Kifc2 in other species, such as Caenorhabditis elegans (Robin et al., 2005) and Saccharomyces cerevisiae (Maddox, 2005), but their roles in vesicle trafficking have not been examined. The present study shows that function of these motors may be substantially different from species to species, likely depending on differential interaction with other vesicle-associated proteins. It is also of interest that early endocytic vesicles do not use dynein for minus-end motility. Rather, our previous studies showed that dynein mediates minus-end motility of late endocytic vesicles (Bananis et al., 2004). Presumably, interaction with dynein requires binding to specific vesicle-associated proteins that are not present on early endocytic vesicles. Proteomic analysis of specific populations of endocytic vesicles may help to identify and characterize these proteins (Bananis et al., 2004).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Lawrence S.B. Goldstein for kindly providing Kifc2 knockout mice, Kifc2 cDNA, and antibody to Kif5B and Dr. Erika L.F. Holzbaur for providing KLC2 cDNA. We also thank Dr. Brigid Joseph (Albert Einstein College of Medicine) and David S. Neufeld (Albert Einstein College of Medicine) for assistance with hepatocyte isolation. This work was supported by National Institutes of Health Grants DK-41918 (to A.W.W.) and GM-60628 (to A.O.S.).
Abbreviations used:
- AMP-PNP
5′-adenylylimido-diphosphate
- ASGPR
asialoglycoprotein receptor
- ASOR
asialoorosomucoid.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-06-0524) on March 14, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Bananis E., Murray J. W., Stockert R. J., Satir P., Wolkoff A. W. Microtubule and motor-dependent endocytic vesicle sorting in vitro. J. Cell Biol. 2000;151:179–186. doi: 10.1083/jcb.151.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bananis E., Murray J. W., Stockert R. J., Satir P., Wolkoff A. W. Regulation of early endocytic vesicle motility and fission in a reconstituted system. J. Cell Sci. 2003;116:2749–2761. doi: 10.1242/jcs.00478. [DOI] [PubMed] [Google Scholar]
- Bananis E., Nath S., Gordon K., Satir P., Stockert R. J., Murray J. W., Wolkoff A. W. Microtubule-dependent movement of late endocytic vesicles in vitro: requirements for dynein and kinesin. Mol. Biol. Cell. 2004;15:3688–3697. doi: 10.1091/mbc.E04-04-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady S. T., Pfister K. K., Bloom G. S. A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc. Natl. Acad. Sci. USA. 1990;87:1061–1065. doi: 10.1073/pnas.87.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou A., Lederer C. W., Surrey T., Vernos I., Santama N. Motor protein KIFC5A interacts with Nubp1 and Nubp2, and is implicated in the regulation of centrosome duplication. J. Cell Sci. 2006;119:2035–2047. doi: 10.1242/jcs.02922. [DOI] [PubMed] [Google Scholar]
- Driskell O. J., Mironov A., Allan V. J., Woodman P. G. Dynein is required for receptor sorting and the morphogenesis of early endosomes. Nat. Cell Biol. 2007;9:113–120. doi: 10.1038/ncb1525. [DOI] [PubMed] [Google Scholar]
- Goltz J. S., Wolkoff A. W., Novikoff P. M., Stockert R. J., Satir P. A role for microtubules in sorting endocytic vesicles in rat hepatocytes. Proc. Natl. Acad. Sci. USA. 1992;89:7026–7030. doi: 10.1073/pnas.89.15.7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G., Nedelec F., Vale R. D. Mechanisms for focusing mitotic spindle poles by minus end-directed motor proteins. J. Cell Biol. 2005;171:229–240. doi: 10.1083/jcb.200505107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon D. W., Yang Z., Goldstein L. S. Characterization of KIFC2, a neuronal kinesin superfamily member in mouse. Neuron. 1997;18:439–451. doi: 10.1016/s0896-6273(00)81244-1. [DOI] [PubMed] [Google Scholar]
- Harford J., Bridges K., Ashwell G., Klausner R. D. Intracellular dissociation of receptor-bound asialoglycoproteins in cultured hepatocytes. A pH-mediated nonlysosomal event. J. Biol. Chem. 1983a;258:3191–3197. [PubMed] [Google Scholar]
- Harford J., Wolkoff A. W., Ashwell G., Klausner R. D. Monensin inhibits intracellular dissociation of asialoglycoproteins from their receptor. J. Cell Biol. 1983b;96:1824–1828. doi: 10.1083/jcb.96.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. K., McMahon H. T. Snap-shots of clathrin-mediated endocytosis. Trends Biochem. Sci. 2002;27:257–263. doi: 10.1016/s0968-0004(02)02089-3. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- Hoang E., Bost-Usinger L., Burnside B. Characterization of a novel C-kinesin (KIFC3) abundantly expressed in vertebrate retina and RPE. Exp. Eye Res. 1999;69:57–68. doi: 10.1006/exer.1999.0671. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Martensen T., Nath J., Flavin M. Inhibition of dynein ATPase by vanadate, and its possible use as a probe for the role of dynein in cytoplasmic motility. Biochem. Biophys. Res. Commun. 1978;81:1313–1318. doi: 10.1016/0006-291x(78)91279-2. [DOI] [PubMed] [Google Scholar]
- Lawrence C. J., et al. A standardized kinesin nomenclature. J. Cell Biol. 2004;167:19–22. doi: 10.1083/jcb.200408113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. E., Murray J. W., Wolkoff A. W., Wilson D. W. Reconstitution of herpes simplex virus microtubule-dependent trafficking in vitro. J. Virol. 2006;80:4264–4275. doi: 10.1128/JVI.80.9.4264-4275.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon L. A., Tokito M., Finklestein J. M., Grossman F. E., Holzbaur E. L. A direct interaction between cytoplasmic dynein and kinesin I may coordinate motor activity. J. Biol. Chem. 2004;279:19201–19208. doi: 10.1074/jbc.M313472200. [DOI] [PubMed] [Google Scholar]
- Macia E., Ehrlich M., Massol R., Boucrot E., Brunner C., Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Maddox P. S. Microtubules: Kar3 eats up the track. Curr. Biol. 2005;15:R622–R624. doi: 10.1016/j.cub.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Marsh M., McMahon H. T. The structural era of endocytosis. Science. 1999;285:215–220. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- Martin M., Iyadurai S. J., Gassman A., Gindhart J. G., Jr, Hays T. S., Saxton W. M. Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol. Biol. Cell. 1999;10:3717–3728. doi: 10.1091/mbc.10.11.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R., McGraw T. E. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Miki H., Okada Y., Hirokawa N. Analysis of the kinesin superfamily: insights into structure and function. Trends Cell Biol. 2005;15:467–476. doi: 10.1016/j.tcb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Miki H., Setou M., Hirokawa N. Kinesin superfamily proteins (KIFs) in the mouse transcriptome. Genome Res. 2003;13:1455–1465. doi: 10.1101/gr.984503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki H., Setou M., Kaneshiro K., Hirokawa N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc. Natl. Acad. Sci. USA. 2001;98:7004–7011. doi: 10.1073/pnas.111145398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Ghosh R. N., Maxfield F. R. Endocytosis. Physiol. Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Murray J. W., Bananis E., Wolkoff A. W. Reconstitution of ATP-dependent movement of endocytic vesicles along microtubules in vitro: an oscillatory bidirectional process. Mol. Biol. Cell. 2000;11:419–433. doi: 10.1091/mbc.11.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. W., Bananis E., Wolkoff A. W. Immunofluorescence microchamber technique for characterizing isolated organelles. Anal. Biochem. 2002;305:55–67. doi: 10.1006/abio.2002.5655. [DOI] [PubMed] [Google Scholar]
- Murray J. W., Wolkoff A. W. Roles of the cytoskeleton and motor proteins in endocytic sorting. Adv. Drug Deliv. Rev. 2003;55:1385–1403. doi: 10.1016/j.addr.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Murray J. W., Wolkoff A. W. Assay of Rab4-dependent trafficking on microtubules. Methods Enzymol. 2005;403:92–107. doi: 10.1016/S0076-6879(05)03009-0. [DOI] [PubMed] [Google Scholar]
- Navolanic P. M., Sperry A. O. Identification of isoforms of a mitotic motor in mammalian spermatogenesis. Biol. Reprod. 2000;62:1360–1369. doi: 10.1095/biolreprod62.5.1360. [DOI] [PubMed] [Google Scholar]
- Nielsen E., Severin F., Backer J. M., Hyman A. A., Zerial M. Rab5 regulates motility of early endosomes on microtubules. Nat. Cell Biol. 1999;1:376–382. doi: 10.1038/14075. [DOI] [PubMed] [Google Scholar]
- Noda Y., Okada Y., Saito N., Setou M., Xu Y., Zhang Z., Hirokawa N. KIFC3, a microtubule minus end-directed motor for the apical transport of annexin XIIIb-associated Triton-insoluble membranes. J. Cell Biol. 2001;155:77–88. doi: 10.1083/jcb.200108042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff P. M., Cammer M., Tao L., Oda H., Stockert R. J., Wolkoff A. W., Satir P. Three-dimensional organization of rat hepatocyte cytoskeleton: relation to the asialoglycoprotein endocytosis pathway. J. Cell Sci. 1996;109:21–32. doi: 10.1242/jcs.109.1.21. [DOI] [PubMed] [Google Scholar]
- Perrais D., Merrifield C. J. Dynamics of endocytic vesicle creation. Dev. Cell. 2005;9:581–592. doi: 10.1016/j.devcel.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Rahman A., Friedman D. S., Goldstein L. S. Two kinesin light chain genes in mice. Identification and characterization of the encoded proteins. J. Biol. Chem. 1998;273:15395–15403. doi: 10.1074/jbc.273.25.15395. [DOI] [PubMed] [Google Scholar]
- Robin G., DeBonis S., Dornier A., Cappello G., Ebel C., Wade R. H., Thierry-Mieg D., Kozielski F. Essential kinesins: characterization of Caenorhabditis elegans KLP-15. Biochemistry. 2005;44:6526–6536. doi: 10.1021/bi048157h. [DOI] [PubMed] [Google Scholar]
- Saito N., Okada Y., Noda Y., Kinoshita Y., Kondo S., Hirokawa N. KIFC2 is a novel neuron-specific C-terminal type kinesin superfamily motor for dendritic transport of multivesicular body-like organelles. Neuron. 1997;18:425–438. doi: 10.1016/s0896-6273(00)81243-x. [DOI] [PubMed] [Google Scholar]
- Samuelson A. C., Stockert R. J., Novikoff A. B., Novikoff P. M., Saez J. C., Spray D. C., Wolkoff A. W. Influence of cytosolic pH on receptor-mediated endocytosis of asialoorosomucoid. Am. J. Physiol. 1988;254:C829–C838. doi: 10.1152/ajpcell.1988.254.6.C829. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Bananis E., Nath S., Anwer M. S., Wolkoff A. W., Murray J. W. PKCzeta is required for microtubule-based motility of vesicles containing the ntcp transporter. Traffic. 2006;7:1078–1091. doi: 10.1111/j.1600-0854.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- Smith M. J., Pozo K., Brickley K., Stephenson F. A. Mapping the GRIF-1 binding domain of the kinesin, KIF5C, substantiates a role for GRIF-1 as an adaptor protein in the anterograde trafficking of cargoes. J. Biol. Chem. 2006;15:27216–27228. doi: 10.1074/jbc.M600522200. [DOI] [PubMed] [Google Scholar]
- Stenoien D. L., Brady S. T. Immunochemical analysis of kinesin light chain function. Mol. Biol. Cell. 1997;8:675–689. doi: 10.1091/mbc.8.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert R. J., Haimes H. B., Morell A. G., Novikoff P. M., Novikoff A. B., Quintana N., Sternlieb I. Endocytosis of asialoglycoprotein-enzyme conjugates by hepatocytes. Lab. Investig. 1980;43:556–563. [PubMed] [Google Scholar]
- Theiss C., Napirei M., Meller K. Impairment of anterograde and retrograde neurofilament transport after anti-kinesin and anti-dynein antibody microinjection in chicken dorsal root ganglia. Eur. J. Cell Biol. 2005;84:29–43. doi: 10.1016/j.ejcb.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Treichel U., Paietta E., Poralla T., Meyer zum Buschenfelde K. H., Stockert R. J. Effects of cytokines on synthesis and function of the hepatic asialoglycoprotein receptor. J. Cell Physiol. 1994;158:527–534. doi: 10.1002/jcp.1041580319. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Malik F., Brown D. Directional instability of microtubule transport in the presence of kinesin and dynein, two opposite polarity motor proteins. J. Cell Biol. 1992;119:1589–1596. doi: 10.1083/jcb.119.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey K. J., Meyer D., Deehan R., Blenis J., Schnapp B. J., Rapoport T. A., Margolis B. Cargo of kinesin identified as jip scaffolding proteins and associated signaling molecules. J. Cell Biol. 2001;152:959–970. doi: 10.1083/jcb.152.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer C. M., Karki S. B., Kuznetsov S. A., Tabb J. S., Weiss D. G., Langford G. M., Holzbaur E. L. The interaction between cytoplasmic dynein and dynactin is required for fast axonal transport. Proc. Natl. Acad. Sci. USA. 1997;94:12180–12185. doi: 10.1073/pnas.94.22.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkoff A. W., Klausner R. D., Ashwell G., Harford J. Intracellular segregation of asialoglycoproteins and their receptor: a prelysosomal event subsequent to dissociation of the ligand-receptor complex. J. Cell Biol. 1984;98:375–381. doi: 10.1083/jcb.98.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Jones B. E., Neufeld D. S., Czaja M. J. Glutathione modulates rat and mouse hepatocyte sensitivity to tumor necrosis factor toxicity. Gastroenterology. 1998;115:1229–1237. doi: 10.1016/s0016-5085(98)70095-2. [DOI] [PubMed] [Google Scholar]
- Xu Y., Takeda S., Nakata T., Noda Y., Tanaka Y., Hirokawa N. Role of KIFC3 motor protein in Golgi positioning and integration. J. Cell Biol. 2002;158:293–303. doi: 10.1083/jcb.200202058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. X., Jefferson H., Sperry A. O. The molecular motor KIFC1 associates with a complex containing nucleoporin NUP62 that is regulated during development and by the small GTPase RAN. Biol. Reprod. 2006;74:684–690. doi: 10.1095/biolreprod.105.049312. [DOI] [PubMed] [Google Scholar]
- Yang W. X., Sperry A. O. C-Terminal kinesin motor KIFC1 participates in acrosome biogenesis and vesicle transport. Biol. Reprod. 2003;69:1719–1729. doi: 10.1095/biolreprod.102.014878. [DOI] [PubMed] [Google Scholar]
- Yang Z., Goldstein L. S. Characterization of the KIF3C neural kinesin-like motor from mouse. Mol. Biol. Cell. 1998;9:249–261. doi: 10.1091/mbc.9.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Hanlon D. W., Marszalek J. R., Goldstein L. S. Identification, partial characterization, and genetic mapping of kinesin-like protein genes in mouse. Genomics. 1997;45:123–131. doi: 10.1006/geno.1997.4901. [DOI] [PubMed] [Google Scholar]
- Yang Z., Roberts E. A., Goldstein L. S. Functional analysis of mouse C-terminal kinesin motor KifC2. Mol. Cell Biol. 2001a;21:2463–2466. doi: 10.1128/MCB.21.7.2463-2466.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Xia C., Roberts E. A., Bush K., Nigam S. K., Goldstein L. S. Molecular cloning and functional analysis of mouse C-terminal kinesin motor KifC3. Mol. Cell Biol. 2001b;21:765–770. doi: 10.1128/MCB.21.3.765-770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Sperry A. O. Comparative analysis of two C-terminal kinesin motor proteins: KIFC1 and KIFC5A. Cell Motil. Cytoskeleton. 2004;58:213–230. doi: 10.1002/cm.20008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.