Abstract
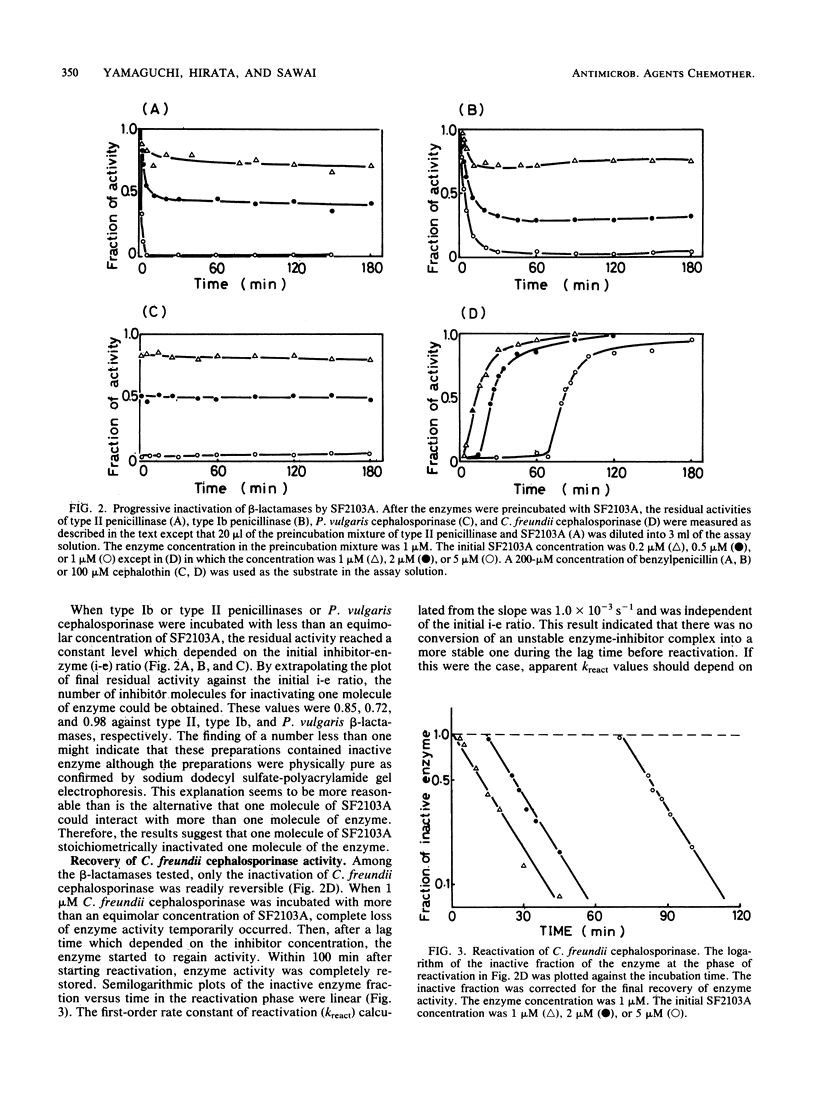
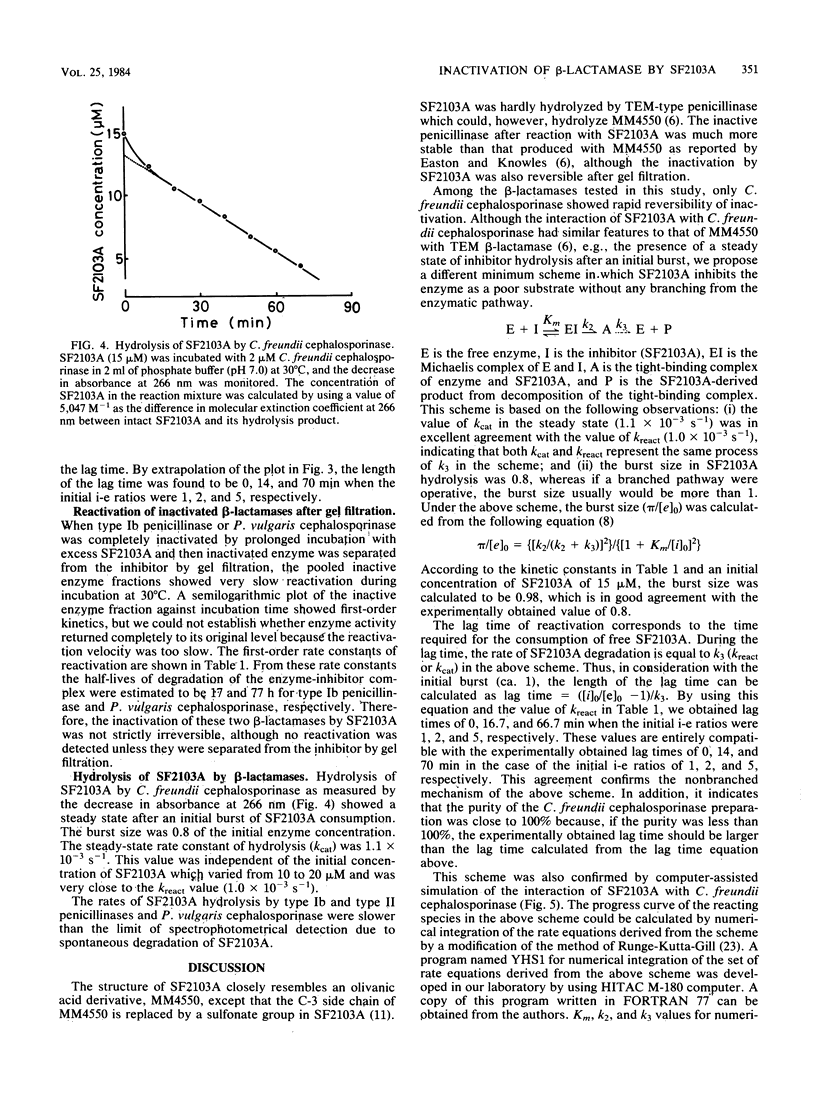
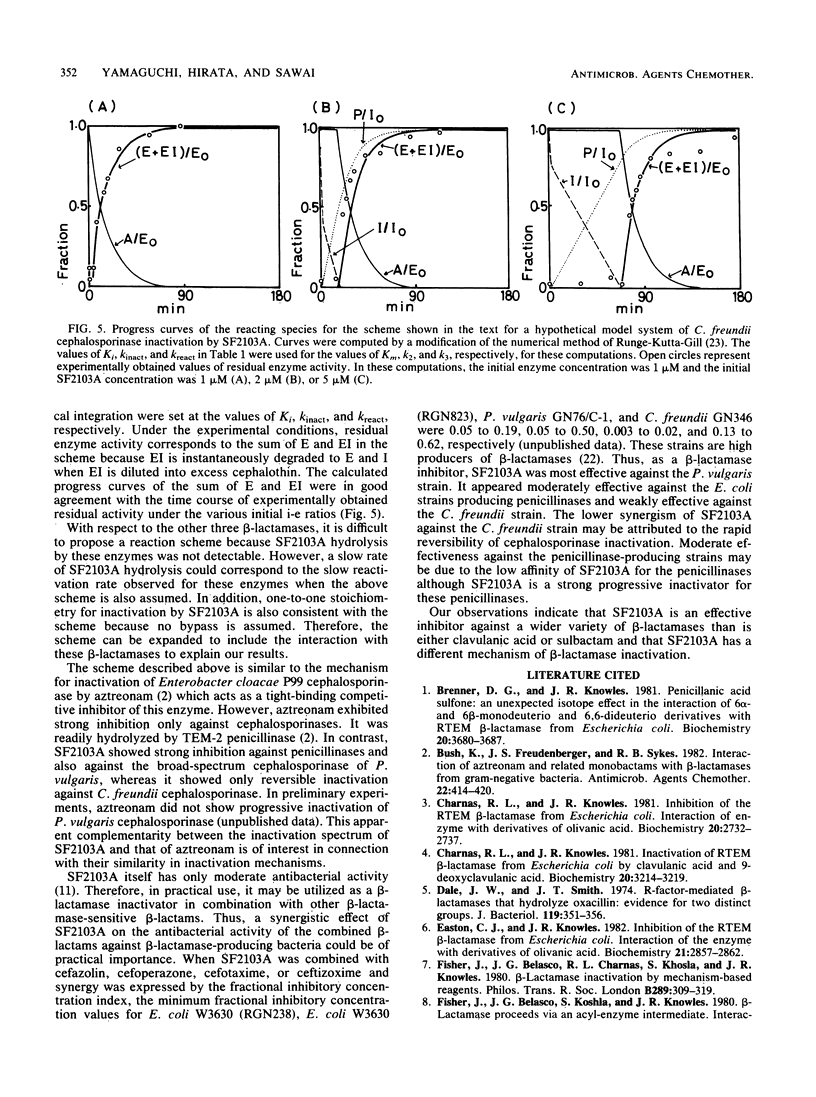
A novel carbapenem, SF2103A, is a strong inhibitor of various types of beta-lactamase. Equimolar concentrations of SF2103A completely inactivated the cephalosporinases of Proteus vulgaris and Citrobacter freundii and type Ib and type II penicillinases mediated by R plasmids in a progressive manner. The inactivation of the two penicillinases and P. vulgaris cephalosporinase was apparently irreversible; however, when the inactivated enzymes were separated from excess SF2103A by gel filtration, they showed very slow reactivation. The hydrolysis of SF2103A by these three beta-lactamases was below the limit of detection. It is concluded that SF2103A acts as a tight-binding competitive inhibitor for the penicillinases and P. vulgaris cephalosporinase. In contrast, the inactivation of C. freundii cephalosporinase by SF2103A was evidently reversible. The rate constant of reactivation of the enzyme was compatible with the turnover rate of the enzyme in the steady state of SF2103A hydrolysis. Thus, SF2103A simply acts as a poor substrate for C. freundii cephalosporinase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner D. G., Knowles J. R. Penicillanic acid sulfone: an unexpected isotope effect in the interaction of 6 alpha- and 6 beta-monodeuterio and of 6,6-dideuterio derivatives with RTEM beta-lactamase from Escherichia coli. Biochemistry. 1981 Jun 23;20(13):3680–3687. doi: 10.1021/bi00516a003. [DOI] [PubMed] [Google Scholar]
- Bush K., Freudenberger J. S., Sykes R. B. Interaction of azthreonam and related monobactams with beta-lactamases from gram-negative bacteria. Antimicrob Agents Chemother. 1982 Sep;22(3):414–420. doi: 10.1128/aac.22.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnas R. L., Knowles J. R. Inactivation of RTEM beta-lactamase from Escherichia coli by clavulanic acid and 9-deoxyclavulanic acid. Biochemistry. 1981 May 26;20(11):3214–3219. doi: 10.1021/bi00514a035. [DOI] [PubMed] [Google Scholar]
- Charnas R. L., Knowles J. R. Inhibition of the RTEM beta-lactamase from Escherichia coli. Interaction of enzyme with derivatives of olivanic acid. Biochemistry. 1981 May 12;20(10):2732–2737. doi: 10.1021/bi00513a005. [DOI] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. R-factor-mediated beta-lactamases that hydrolyze oxacillin: evidence for two distinct groups. J Bacteriol. 1974 Aug;119(2):351–356. doi: 10.1128/jb.119.2.351-356.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton C. J., Knowles J. R. Inhibition of the RTEM beta-lactamase from Escherichia coli. Interaction of the enzyme with derivatives of olivanic acid. Biochemistry. 1982 Jun 8;21(12):2857–2862. doi: 10.1021/bi00541a008. [DOI] [PubMed] [Google Scholar]
- Fisher J., Belasco J. G., Charnas R. L., Khosla S., Knowles J. R. Beta-lactamase inactivation by mechanism-based reagents. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):309–319. doi: 10.1098/rstb.1980.0048. [DOI] [PubMed] [Google Scholar]
- Fisher J., Belasco J. G., Khosla S., Knowles J. R. beta-Lactamase proceeds via an acyl-enzyme intermediate. Interaction of the Escherichia coli RTEM enzyme with cefoxitin. Biochemistry. 1980 Jun 24;19(13):2895–2901. doi: 10.1021/bi00554a012. [DOI] [PubMed] [Google Scholar]
- Fisher J., Charnas R. L., Bradley S. M., Knowles J. R. Inactivation of the RTEM beta-lactamase from Escherichia coli. Interaction of penam sulfones with enzyme. Biochemistry. 1981 May 12;20(10):2726–2731. doi: 10.1021/bi00513a004. [DOI] [PubMed] [Google Scholar]
- Fisher J., Charnas R. L., Knowles J. R. Kinetic studies on the inactivation of Escherichia coli RTEM beta-lactamase by clavulanic acid. Biochemistry. 1978 May 30;17(11):2180–2184. doi: 10.1021/bi00604a024. [DOI] [PubMed] [Google Scholar]
- Ito T., Ezaki N., Ohba K., Amano S., Kondo Y., Miyado H. S., Shomura T., Sezaki M., Niwa T., Kojima M. A novel beta-lactamase inhibitor, SF-2103 A produced by a Streptomyces. J Antibiot (Tokyo) 1982 Apr;35(4):533–535. doi: 10.7164/antibiotics.35.533. [DOI] [PubMed] [Google Scholar]
- Labia R., Lelievre V., Peduzzi J. Inhibition kinetics of three R-factor-mediated beta-lactamases by a new beta-lactam sulfone (CP 45899). Biochim Biophys Acta. 1980 Feb 14;611(2):351–357. doi: 10.1016/0005-2744(80)90071-6. [DOI] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W. Analytical isoelectric focusing of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1976 Feb;125(2):713–718. doi: 10.1128/jb.125.2.713-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K., Sakamoto M., Ishikura T. PS-5 inhibition of a beta-lactamase from Proteus vulgaris. J Antibiot (Tokyo) 1980 Mar;33(3):293–302. doi: 10.7164/antibiotics.33.293. [DOI] [PubMed] [Google Scholar]
- RIchmond M. H. The semi-synthetic thienamycin derivative MK0787 and its properties with respect to a range of beta-lactamases from clinically relevant bacterial species. J Antimicrob Chemother. 1981 Mar;7(3):279–285. doi: 10.1093/jac/7.3.279. [DOI] [PubMed] [Google Scholar]
- Reading C., Farmer T. The inhibition of beta-lactamases from gram-negative bacteria by clavulanic acid. Biochem J. 1981 Dec 1;199(3):779–787. doi: 10.1042/bj1990779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Kanno M., Tsukamoto K. Characterization of eight beta-lactamases of Gram-negative bacteria. J Bacteriol. 1982 Nov;152(2):567–571. doi: 10.1128/jb.152.2.567-571.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Nakajima S., Morohoshi T., Yamagishi S. Thermolabile repression of cephalosporinase synthesis in Citrobacter freundii. Microbiol Immunol. 1977 Nov;21(11):631–638. doi: 10.1111/j.1348-0421.1977.tb00331.x. [DOI] [PubMed] [Google Scholar]
- Sawai T., Takahashi K., Yamagishi S., Mitsuhashi S. Variant of penicillinase mediated by an R factor in Escherichia coli. J Bacteriol. 1970 Nov;104(2):620–629. doi: 10.1128/jb.104.2.620-629.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Tsukamoto K. Cefoxitin, N-formimidoyl thienamycin, clavulanic acid, and penicillanic acid sulfone as suicide inhibitors for different types of beta-lactamases produced by gram-negative bacteria. J Antibiot (Tokyo) 1982 Nov;35(11):1594–1602. doi: 10.7164/antibiotics.35.1594. [DOI] [PubMed] [Google Scholar]
- Sawai T., Yoshida T., Tsukamoto K., Yamagishi S. A set of bacterial strains for evaluation of beta-lactamase-stability of beta-lactam antibiotics. J Antibiot (Tokyo) 1981 Oct;34(10):1318–1326. doi: 10.7164/antibiotics.34.1318. [DOI] [PubMed] [Google Scholar]
- Tatsunami S., Yago N., Hosoe M. Kinetics of suicide substrates. Steady-state treatments and computer-aided exact solutions. Biochim Biophys Acta. 1981 Dec 15;662(2):226–235. doi: 10.1016/0005-2744(81)90034-6. [DOI] [PubMed] [Google Scholar]
- Yamagishi S., O'Hara K., Sawai T., Mitsuhashi S. The purification and properties of penicillin beta-lactamases mediated by transmissible R factors in Escherichia coli. J Biochem. 1969 Jul;66(1):11–20. doi: 10.1093/oxfordjournals.jbchem.a129111. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A., Hirata T., Sawai T. Kinetic studies on inactivation of Citrobacter freundii cephalosporinase by sulbactam. Antimicrob Agents Chemother. 1983 Jul;24(1):23–30. doi: 10.1128/aac.24.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]


