Abstract
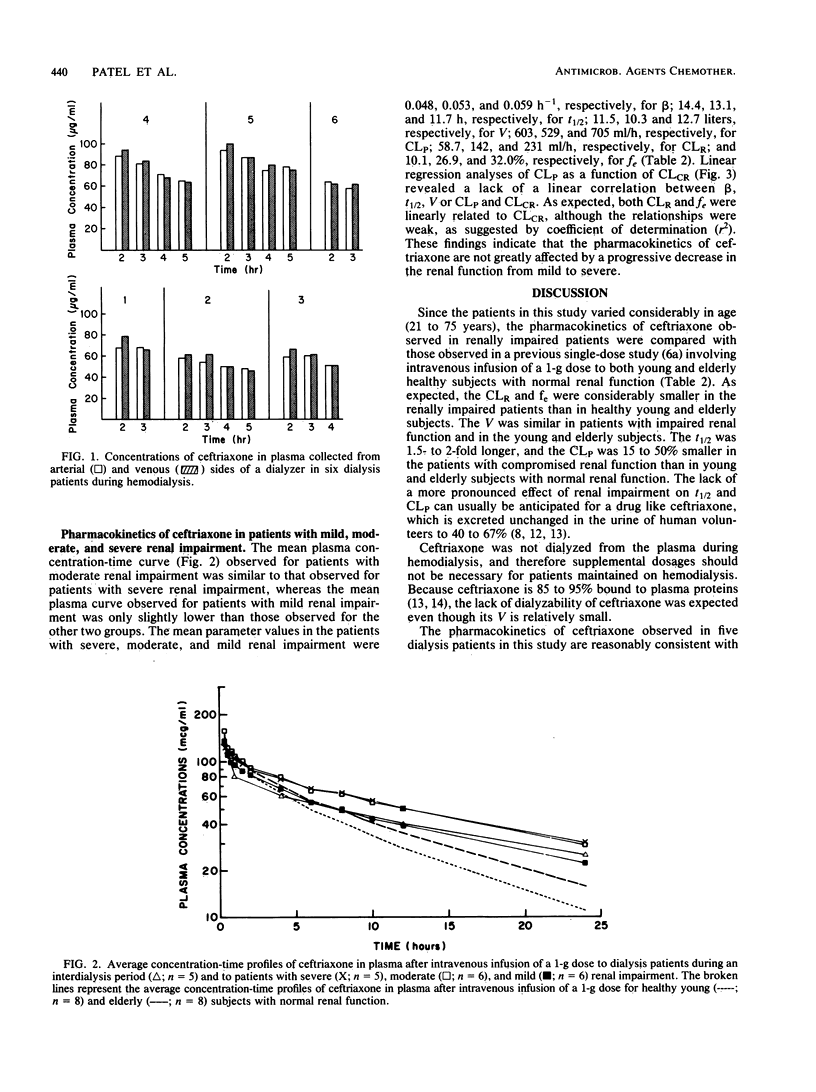
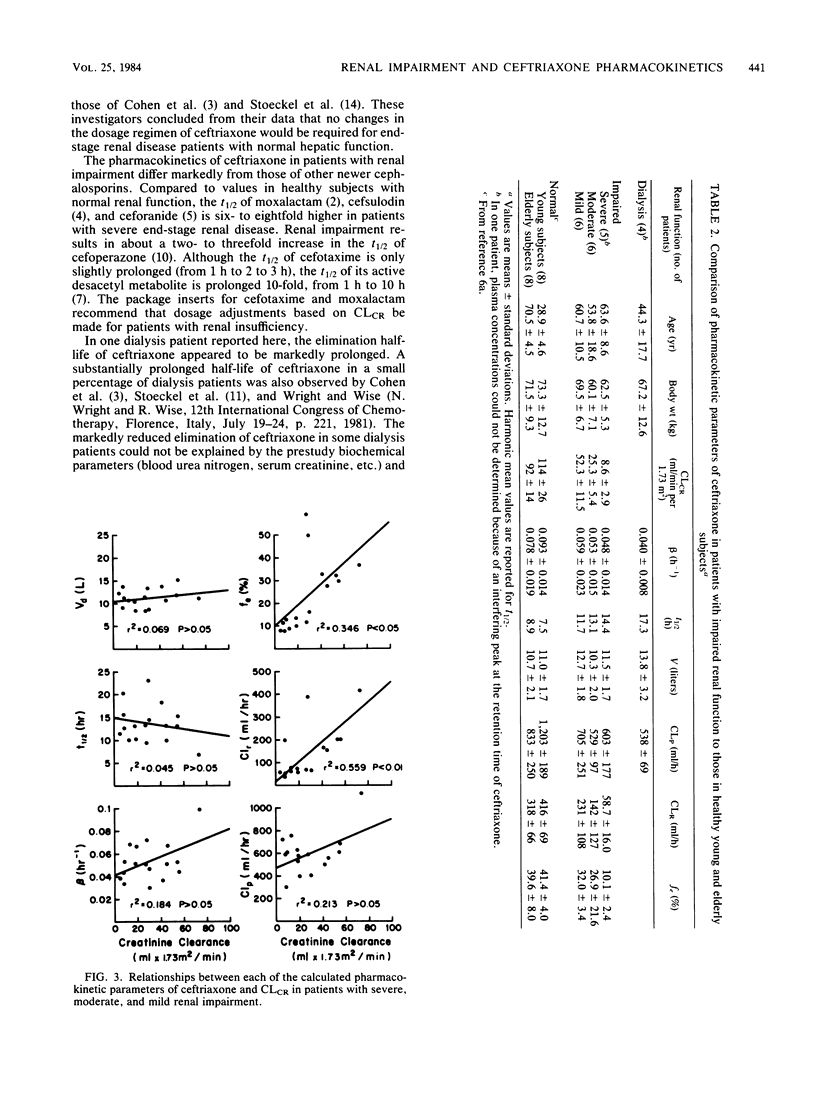
The effects of renal impairment on the pharmacokinetics of ceftriaxone in humans were examined after intravenous infusion of a 1-g dose over 15 min to 30 renally impaired patients. The study included 12 dialysis patients and 18 patients with severe, moderate, or mild renal impairment. Plasma and, where appropriate, urine and dialysate samples were collected at predetermined times and analyzed for ceftriaxone by high-pressure liquid chromatography. The elimination half-life (group mean ranged from 11.7 to 17.3 h) and plasma clearance (group mean ranged from 529 to 705 ml/h) did not correlate linearly with creatinine clearance. The renal clearance and fraction of dose excreted unchanged in urine were related linearly, however weakly, with creatinine clearance. Ceftriaxone was not removed from plasma to a significant extent during hemodialysis. The half-life was prolonged twofold, the plasma clearance was lowered less than 50%, and the volume of distribution was relatively unchanged in renally impaired patients compared with young or elderly healthy subjects with normal renal function at an equivalent dose. Since these changes are moderate, adjustment in the dosage regimen of ceftriaxone for patients with impaired renal function should not be necessary when ceftriaxone dosage is 2 g or less per day (2 g every 24 h or 1 g every 12 h). It was reported that the elimination half-life of ceftriaxone is substantially prolonged in a small percentage of patients with end-stage renal disease maintained on hemodialysis. Therefore, plasma concentrations of ceftriaxone should be monitored in dialysis patients to determine whether dosage adjustments are necessary.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angehrn P., Probst P. J., Reiner R., Then R. L. Ro 13-9904, a long-acting broad-spectrum cephalosporin: in vitro and in vivo studies. Antimicrob Agents Chemother. 1980 Dec;18(6):913–921. doi: 10.1128/aac.18.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton W. K., Scheld W. M., Spyker D. A., Overby T. L., Sande M. A. Pharmacokinetics of moxalactam in subjects with various degrees of renal dysfunction. Antimicrob Agents Chemother. 1980 Dec;18(6):933–938. doi: 10.1128/aac.18.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D., Appel G. B., Scully B., Neu H. C. Pharmacokinetics of ceftriaxone in patients with renal failure and in those undergoing hemodialysis. Antimicrob Agents Chemother. 1983 Oct;24(4):529–532. doi: 10.1128/aac.24.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson T. P., Granneman G. R., Kallal J. E., Sennello L. T. Cefsulodin kinetics in renal impairment. Clin Pharmacol Ther. 1982 May;31(5):602–608. doi: 10.1038/clpt.1982.84. [DOI] [PubMed] [Google Scholar]
- Hawkins S. S., Alford R. H., Stone W. J., Smyth R. D., Pfeffer M. Ceforanide kinetics in renal insufficiency. Clin Pharmacol Ther. 1981 Oct;30(4):468–474. doi: 10.1038/clpt.1981.190. [DOI] [PubMed] [Google Scholar]
- Holazo A. A., Colburn W. A. Pharmacokinetics of drugs during various detoxification procedures for overdose and environmental exposure. Drug Metab Rev. 1982;13(4):715–743. doi: 10.3109/03602538209011094. [DOI] [PubMed] [Google Scholar]
- Luderer J. R., Patel I. H., Durkin J., Schneck D. W. Age and ceftriaxone kinetics. Clin Pharmacol Ther. 1984 Jan;35(1):19–25. doi: 10.1038/clpt.1984.3. [DOI] [PubMed] [Google Scholar]
- McNamara P. J., Stoeckel K., Ziegler W. H. Pharmacokinetics of ceftriaxone following intravenous administration of a 3 g dose. Eur J Clin Pharmacol. 1982;22(1):71–75. doi: 10.1007/BF00606428. [DOI] [PubMed] [Google Scholar]
- Neu H. C. A review and summary of the pharmacokinetics of cefoperazone: a new, extended-spectrum beta-lactam antibiotic. Ther Drug Monit. 1981;3(2):121–128. doi: 10.1097/00007691-198102000-00002. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Meropol N. J., Fu K. P. Antibacterial activity of ceftriaxone (Ro 13-9904), a beta-lactamase-stable cephalosporin. Antimicrob Agents Chemother. 1981 Mar;19(3):414–423. doi: 10.1128/aac.19.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel I. H., Chen S., Parsonnet M., Hackman M. R., Brooks M. A., Konikoff J., Kaplan S. A. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother. 1981 Nov;20(5):634–641. doi: 10.1128/aac.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel K., McNamara P. J., Brandt R., Plozza-Nottebrock H., Ziegler W. H. Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin Pharmacol Ther. 1981 May;29(5):650–657. doi: 10.1038/clpt.1981.90. [DOI] [PubMed] [Google Scholar]
- Stoeckel K., McNamara P. J., Hoppe-Seyler G., Blumberg A., Keller E. Single-dose ceftriaxone kinetics in functionally anephric patients. Clin Pharmacol Ther. 1983 May;33(5):633–641. doi: 10.1038/clpt.1983.86. [DOI] [PubMed] [Google Scholar]


