Abstract
The synergistic effects of daptomycin plus gentamicin or rifampin were tested against 50 Staphylococcus aureus strains, with daptomycin MICs ranging between 0.25 and 8 μg/ml. Daptomycin sub-MICs combined with gentamicin concentrations lower than the MIC yielded synergy in 34 (68%) of the 50 strains. Daptomycin combined with rifampin yielded synergy in one vancomycin-intermediate S. aureus strain only, and virtually all synergy occurred between daptomycin and gentamicin.
Methicillin-resistant Staphylococcus aureus (MRSA) strains are increasingly encountered and cannot be treated with available β-lactams. Most methicillin-resistant (and also some methicillin-susceptible S. aureus [MSSA]) strains are resistant to all available quinolones, and vancomycin-intermediate (VISA) and vancomycin-resistant (VRSA) S. aureus strains have appeared (3-5, 8, 11, 15, 23). Most multidrug-resistant S. aureus strains are nosocomially acquired and cause an array of site-specific infections in hospitalized patients, including bloodstream infections, pneumonia, surgical-site infections, and urinary tract infections. However, in the past few years there has been an increase in the incidence of community-acquired MRSA which, although at this time susceptible to most other agents, are more virulent than hospital strains (1, 7, 10, 12-14, 18, 21, 22). Although previously considered to play an important role in the virulence of community-acquired MRSA strains, a recent report (25) has cast doubt upon the importance of Panton-Valentine leukocidin production in the pathogenicity of these strains.
The development of S. aureus strains with diminished susceptibility to vancomycin is at least partially caused by the selective pressure of vancomycin use in the community (23). The increase in community-acquired MRSA strains will likely lead to more glycopeptide use in the community setting, therefore increasing the selective pressure for vancomycin resistance. An alternative to glycopeptides is urgently needed.
Daptomycin is very potent against S. aureus, with low MICs, rapid killing, and excellent clinical activity (17, 20). Daptomycin is currently approved in the United States for the treatment of skin and soft tissue infections, S. aureus bacteremia, and right-sided endocarditis (20). Increasing daptomycin MICs have been reported and a possible correlation (based perhaps on the shared activity of both drugs at different sites in a thickened cell envelope) for higher daptomycin MICs in laboratory derived-serial passage isolates has been described (3, 4).
We have used time-kill synergy studies to assess the activity of daptomycin, alone and combination with rifampin and gentamicin, against 50 S. aureus strains with various daptomycin MICs.
The S. aureus isolates included the following: VISA, 6 strains; VRSA, 3 strains; MSSA, 9 strains; and MRSA, 32 strains (20 community-acquired, 15 of these were Panton-Valentine leukocidin positive, and 12 were nosocomially acquired. Strains were isolated from the Hershey Medical Center and the University of Texas Southwestern Medical Center, Dallas, TX. Strains were stored frozen at −70°C in double-strength skim milk (Difco, Inc., Detroit, MI) before testing. Daptomycin was obtained from Cubist Pharmaceuticals, Lexington, MA, and rifampin and gentamicin were from Sigma Chemical Co., Inc., St. Louis, MO.
MICs were predetermined by macrodilution in cation-adjusted Mueller-Hinton broth (BBL Microbiology Systems, Cockeysville, MD) according to standard methodology (19). Daptomycin susceptibility testing was performed in Mueller-Hinton broth adjusted to 50 μg/ml of calcium according to standard methodology. All strains were tested by time-kill methodology with each compound alone as described previously (2). Concentrations (35-μl aliquots of suspensions into 5 ml of broth) at one to two dilutions below the MIC were chosen for synergy testing. Viability counts (100-μl aliquots) in synergy tests were performed at 0, 3, 6, 12, and 24 h in a shaking water bath at 35°C with final inocula of between 5 × 105 and 5 × 106 CFU/ml. Only plates with 30 to 300 colonies were counted. Drug carryover was addressed by dilution as described previously. Synergy was defined as a ≥2 log10 decrease in CFU/ml between the combination and its most active component after 3, 6, 12, and 24 h and the number of surviving organisms in the presence of the combination being ≥2 log10 below the starting inoculum at 0 h. At least one of the drugs had to be present in a concentration which did not significantly affect the growth curve of the test organism when used alone (2).
Results of our study correlated by the strain's resistotype are presented in Table 1. Individual synergy data are available electronically in the supplemental material. The MICs of the drugs alone were 0.25 to 8 μg/ml (daptomycin), 0.004 to >128 μg/ml (rifampin), and 0.5 to 1,024 μg/ml (gentamicin). Six VISA strains had increased daptomycin MICs of 2 to 8 μg/ml. Six strains with rifampin MICs of ≥64 μg/ml and 12 strains with gentamicin MICs of >128 μg/ml were not tested.
TABLE 1.
Combined results of MIC and time-kill synergy tests
| Strain | No. of strains | MIC range (μg/ml)a
|
% of strainsb
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DAP+RIF
|
DAP+GEN
|
|||||||||
| DAP | RIF | GEN | Ant | Add | Syn | Ant | Add | Syn | ||
| MSSA | 9c | 0.25-1 | 0.008-0.016 | 0.5->128 | 0 | 100 | 0 | 0 | 50 | 50 |
| CA-MRSA, PVL+ | 15 | 0.5-1 | 0.004-0.016 | 1-2 | 0 | 100 | 0 | 0 | 33.3 | 66.7 |
| CA-MRSA, PVL− | 5d | 0.5-1 | 0.016->64 | 1->128 | 0 | 100 | 0 | 0 | 25 | 75 |
| HA-MRSA | 12e | 0.25-1 | 0.004-256 | 1-512 | 0 | 100 | 0 | 0 | 40 | 60 |
| VISA | 6f | 2-8 | 0.016->128 | 1-1,024 | 0 | 66.7 | 33.3 | 0 | 33.3 | 66.7 |
| VRSA | 3g | 0.25-1 | 0.008->128 | 64-128 | 0 | 100 | 0 | 0 | 33.3 | 66.7 |
DAP, daptomycin; RIF, rifampin; GEN, gentamicin.
That is, the percentage of strains at the 24-h time point demonstrating the labeled effect. Ant, antagonistic; Add, additive; Syn, synergistic.
Isolate 105 was not tested with the DAP+GEN combination.
Isolate 347 was not tested with the DAP+RIF or the DAP+GEN combination.
Isolates 076, 081, 086, 251, 243, and 181 were not tested with the DAP+GEN combination, and isolate 069 was not tested with either combination.
Isolates 555 and 506 were not tested with the DAP+RIF combination, isolates 507 and 508 were not tested with the DAP+GEN combination, and isolate 504 was not tested with either combination.
Isolate 509 was not tested with the DAP+RIF combination.
Only one VISA isolate showed synergy between daptomycin and rifampin at both 12 h (2 and 0.004 μg/ml) and 24 h (2 and 0.008 μg/ml); all other combinations were additive. At 3 h three strains (one MSSA and two community-acquired MRSA isolates, toxin positive) showed synergy between daptomycin and gentamicin (two strains, 0.125 and 0.5 μg/ml; one strain, 0.25 and 0.25 μg/ml). At 6 h, six community-acquired MRSA toxin-positive strains showed synergy at 0.125 and 0.5 μg/ml; in addition, two MSSA strains showed synergy at 0.125 and 0.25 μg/ml; one VISA strain showed synergy at 1 and 0.5 μg/ml; one VRSA strain showed synergy at 0.06 and 32 μg/ml. At 12 h, synergy was seen in 8 MSSA strains (0.125 and 0.25 μg/ml), 13 community-acquired MRSA strains (11 toxin positive; 0.125 and 0.5 μg/ml); 4 hospital-acquired MRSA strains (0.25 and 0.25 μg/ml), 3 VISA strains (1 and 0.5 μg/ml), and three VRSA strains (0.06 and 32 μg/ml). Synergy was seen at 24 h in 4 MSSA strains (0.25 and 0.125 μg/ml), 13 community-acquired MRSA strains (10 toxin positive) (0.125 and 1 μg/ml), 3 hospital-acquired MRSA strains (0.25 and 0.5 μg/ml), 2 VISA strains (2 and 0.5 μg/ml), and 2 VRSA strains (0.06 and 32 μg/ml). All other combinations with daptomycin and gentamicin at all time periods were additive, and no antagonism was found.
Of the nine MSSA strains tested 12% (one of eight strains), 25% (two of eight strains), 100% (all eight strains), and 50% (four of eight strains) showed synergy with daptomycin and gentamicin at 3, 6, 12, and 24 h, respectively. Of the 20 community-acquired MRSA strains tested 11% (2 of 19 strains), 32% (6 of 19 strains) at both 3 and 6 h, and 68% (13 of 19 strains) at both 12 and 24 h showed synergy with daptomycin and gentamicin at sub-MIC combinations. Synergy was also found in 80% (four of five strains) and 60% (three of five strains) at both 12 and 24 h in 12 hospital-acquired MRSA with daptomycin plus gentamicin. The six VISA strains demonstrated synergy with daptomycin and gentamicin with 67% (two of three strains) at both 6 and 24 h and 100% (three of three strains) at 12 h. One of the three VISA strains (33%) showed synergy with daptomycin plus rifampin at both 12 and 24 h. Of the three VRSA strains tested with daptomycin plus gentamicin 33% (one of three strains), 100% (three of three strains), and 67% (two of three strains) showed synergy at 6, 12, and 24 h, respectively.
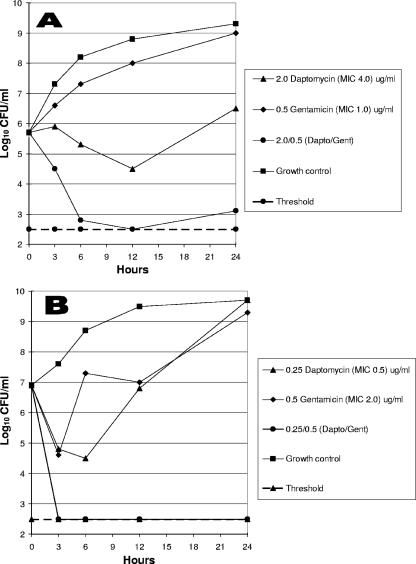
Synergy time-kill graphs for one VISA strain and one community-acquired MRSA strain are depicted graphically in Fig. 1.
FIG. 1.
(A) Daptomycin-gentamicin against a VISA strain; (B) daptomycin-gentamicin against a community-acquired MRSA strain.
Laplante and Rybak (16) evaluated the impact of high-inoculum Staphylococcus aureus (9.5 log10 CFU/g) on the activities of daptomycin, alone and in combination with gentamicin in an in vitro pharmacodynamic model with simulated endocardial vegetations over 72 h. In both strains tested (one MSSA strain and one MRSA strain), the addition of gentamicin increased the rate of 99.9% kill to 8 h for daptomycin (P < 0.01). Tsuji and Rybak (24) have reported that a single dose of gentamicin (5 mg/kg) in combination with daptomycin may be of use to maximize synergistic and bactericidal activity and minimize toxicity, in an in vitro pharmacodynamic model. In contrast, a recent study by DeRyke et al. (9) showed that the coadministration of gentamicin did not alter daptomycin pharmacokinetics. Daptomycin retained bactericidal activity in the presence of gentamicin against most strains except for Enterococcus faecium. No instances of antagonism were observed. Results of combinations between daptomycin and other aminoglycosides have not, to our knowledge, been reported, and the toxicity of both combinations at sub-MICs is not expected to increase. The daptomycin Cmax for skin and soft tissue infections at an approved dose of 4 mg/kg is 57.8 μg/ml and that for bacteremia-right-sided endocarditis at a dose of 6 mg/kg is 98.6 μg/ml (20). At a gentamicin dose of 2 mg/kg the Cmax values in healthy volunteers were 10.1 ± 1.3 μg/ml (predistribution peak) and 6.2 ± 0.4 μg/ml (postdistribution peak), and at a gentamicin dose of 7 mg/kg the Cmax values in healthy volunteers were 39.8 ± 4.1 μg/ml (predistribution peak) and 11.0 ± 0.6 μg/ml (postdistributional peak) (6). More work is necessary before the clinical utility of the current findings can be fully assessed. Although synergy time-kill studies are beyond the capability of most routine laboratories, the Etest method (AB Biodisk, Solna, Swden) may be used for this purpose.
Although daptomycin is uniformly active, as well as rapidly bactericidal, against the vast majority of S. aureus strains encountered clinically (17, 20), recent studies (3, 4) point to a slightly higher daptomycin MIC for some VISA strains, and it is speculated that this may be because of abnormalities in the cell envelope. The present study shows that at 6, 12, or 24 h significant synergy was obtained in vitro between sub-MIC concentrations of daptomycin and gentamicin. Clinical studies will be required to test this hypothesis.
Supplementary Material
Acknowledgments
This study was supported by a grant from Cubist Pharmaceuticals, Lexington, MA.
We thank George McCracken and Susanna Chavez-Bueno for kindly providing some of the strains tested in this study.
Footnotes
Published ahead of print on 12 January 2007.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Adern, P. V., C. P. Montgomery, A. N. Husain, T. K. Koogler, V. Arangelovich, M. Humilier, S. Boyle-Vavra, and R. S. Daum. 2005. Staphylococcus aureus sepsis and the Waterhouse-Freiderichsen syndrome in children. N. Engl. J. Med. 353:1245-1251. [DOI] [PubMed] [Google Scholar]
- 2.Clark, C. L., M. R. Jacobs, and P. C. Appelbaum. 1999. Activities of clinafloxacin, alone and in combination with other compounds, against 45 gram-positive and -negative organisms for which clinafloxacin MICs are high. Antimicrob. Agents Chemother. 43:2295-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui, L., A. Iwamoto, J.-Q. Lian, H.-M. Neoh, T. Murayama, Y. Horikawa, and K. Hiramatsu. 2006. Novel mechanism of antibiotic resistance originating in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50:428-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui, L., E. Tominaga, H.-M. Neoh, and K. Hiramatsu. 2006. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50:1079-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Lassence, A., N. Hidri, J.-F. Timsit, M.-L. Joly-Guillou, G. Thiery, A. Boyer, P. Lable, A. Blivet, H. Kalinowski, Y. Martin, J.-P. Lajonchere, and D. Dreyfuss. Control and outcome of a large outbreak of colonization and infection with glycopeptide-intermediate Staphylococcus aureus in an intensive care unit. Clin. Infect. Dis. 42:170-178. [DOI] [PubMed]
- 6.Demczar, D. J., A. N. Nafziger, and J. S. Bertino, Jr. 1997. Pharmacokinetics of gentamicin at traditional versus high doses: implications for once-daily aminoglycoside dosing. Antimicrob. Agents Chemother. 41:1115-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denis, O., A. Deplano, H. de Beenhouwer, M. Hallin, G. Huysmans, M. G. Garrino, Y. Glupczynski, X. Malaviolle, A. Vergison, and M. J. Streulens. 2005. Polyclonal emergence and importation of community-acquired methicillin-resistant Staphylococcus aureus strains harbouring Panton-Valentin leucocidin genes in Belgium. J. Antimicrob. Chemother. 56:1103-1106. [DOI] [PubMed] [Google Scholar]
- 8.Deresinski, S. 2005. Methicillin-resistant Staphylococcus aureus: an evolutionary, epidemiologic, and therapeutic odyssey. Clin. Infect. Dis. 40:562-573. [DOI] [PubMed] [Google Scholar]
- 9.DeRyke, C. A., C. Sutherland, B. Zhang, D. P. Nicolau, and J. L. Kuti. 2006. Serum bactericidal activities of high-dose daptomycin with or without coadministration of gentamicin against isolates of Staphylococcus aureus and Enterococcus species. Antimicrob. Agents Chemother. 50:3529-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis, J. S., M. C. Doherty, U. Lopatin, C. P. Johnston, G. Sinha, T. Ross, M. Cai, N. N. Hansel, T. Perl, J. R. Ticehurst, K. Carroll, D. L. Thomas, E. Nuermberger, and J. G. Bartlett. 2005. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin. Infect. Dis. 40:100-107. [DOI] [PubMed] [Google Scholar]
- 11.Garnier, F., D. Chainier, T. Walsh, A. Karlsson, A. Bolmström, C. Grelaud, M. Mounier, F. Denis, and M.-C. Ploy. 2006. A 1 year surveillance study of glycopeptide-intermediate Staphylococcus aureus strains in a French hospital. J. Antimicrob. Chemother. 57:146-149. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez, B. E., K. G. Hulten, M. K. Dishop, L. B. Lamberth, W. A. Hammerman, E. O. Mason, Jr., and S. L. Kaplan. 2005. Pulmonary manifestations in children with invasive community-acquired Staphylococcus aureus. Clin. Infect. Dis. 41:583-590. [DOI] [PubMed] [Google Scholar]
- 13.Healy, C. M., K. G. Hulten, D. L. Palazzi, J. R. Campbell, and C. J. Baker. 2004. Emergence of new strains of methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. Clin. Infect. Dis. 39:1460-1466. [DOI] [PubMed] [Google Scholar]
- 14.Kazakova, S. V., J. C. Hageman, M. Matava, A. Srinivasan, L. Phelan, B. Garfinkel, T. Boo, S. McAllister, J. Anderson, B. Jensen, D. Dodson, D. Lonsway, L. K. McDougal, M. Arduino, V. J. Fraser, G. Killgore, F. C. Tenover, S. Cody, and D. B. Jernigan. 2005. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N. Engl. J. Med. 352:468-475. [DOI] [PubMed] [Google Scholar]
- 15.LaPlante, K. L., and M. J. Rybak. 2004. Clinical glycopeptide-intermediate staphylococci tested against arbekacin, daptomycin, and tigecycline. Diagn. Microbiol. Infect. Dis. 50:125-130. [DOI] [PubMed] [Google Scholar]
- 16.Laplante, K. L., and M. J. Rybak. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipsky, B. A., and U. Stoutenburgh. 2005. Daptomycin for treating infected diabetic foot ulcers: evidence from a randomized, controlled trial comparing daptomycin with vancomycin or semi-synthetic penicillin for complicated skin and skin-structure infections. J. Antimicrob. Chemother. 55:240-245. [DOI] [PubMed] [Google Scholar]
- 18.Miller, L. G., F. Perdreau-Remington, G. Rieg, S., Mehdi, J. Perlroth. A. S. Bayer, A. W. Tang, T. O. Phung, and B. Spellberg. 2005. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 352:1445-1453. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 2005. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. NCCLS publication no. M7-A7. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 20.Steenbergen, J., J. Alder, G. M. Thorne, and F. P. Tally. 2005. Daptomycin: a lipopeptide antibiotic for the treatment of serious gram-positive infect6ions. J. Antimicrob. Chemother. 55:283-288. [DOI] [PubMed] [Google Scholar]
- 21.Takizawa, Y., I. Taneike, S. Nakagawa, T. Oishi, Y. Nitahara, N. Iwakura, K. Ozaki, M. Takano, T. Nakayama, and T. Yamamoto. 2005. A Panton-Valentine Leukocidin (PVL)-positive community-acquired methicillin-resistant Staphylococcus aureus (MRSA) strain, another such strain carrying a multiple-drug resistance plasmid, and other more-typical PVL-negative MRSA strains found in Japan. Antimicrob. Agents Chemother. 43:3356-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenover, F. C., L. K. McDougal, R. V. Goering, G. Killgore, S. J. Projan, J. B. Patel, and P. M. Dunman. 2006. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. Antimicrob. Agents Chemother. 44:108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenover, F. C., L. M. Weigel, P. C. Appelbaum, L. K. McDougal, J. Chaitram, S. McAllister, N. Clark, G. Killgore, C. M. O'Hara, L. Jevitt, J. B. Patel, and B. Bozdogan. 2004. Vancomycin-resistant Staphylococcus aureus isolate from a patient in Pennsylvania. Antimicrob. Agents Chemother. 48:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuji, B. T., and M. J. Rybak. 2005. Short-course gentamicin in combination with daptomycin or vancomycin against Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 49:2735-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voyich, J. M., M. Otto, B. Mathema, K. R. Braughton, A. R. Whitney, D. Welty, R. D. Long, D. W. Dorward, D. J. Gardner, G. Lina, B. N. Kreiswirth, and F. R. DeLeo. 2006. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J. Infect. Dis. 194:1761-1770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.