Abstract
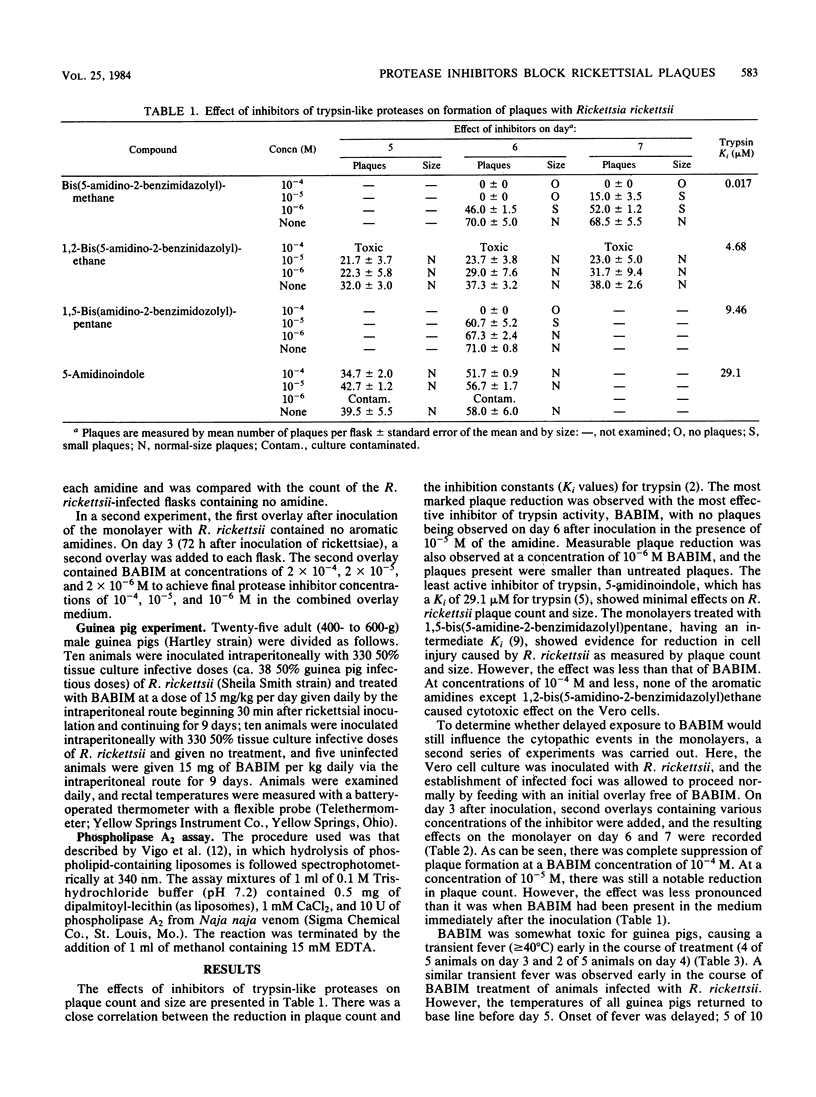
To evaluate the importance of proteolytic activity in the pathogenesis of cell injury by Rickettsia rickettsii, a series of four aromatic amidine inhibitors of trypsin-like proteases were introduced into the plaque model. The compounds were shown to be active toward plaque reduction with their order of effectiveness parallel to their antitrypsin activity. One of the compounds, bis(5-amidino-2-benzimidazolyl)-methane, at a concentration of 10(-5) M demonstrated complete inhibition of plaque formation on day 6. Bis(5-amidino-2-benzimidazolyl)methane at the same concentration reduced cell injury even when added to the system after 72 h of rickettsial infection. The reduction in morbidity in guinea pigs experimentally infected with R. rickettsii and treated with bis(5-amidino-2-benzimidazolyl)methane as compared with morbidity in infected, untreated animals, comprised delay in the onset of fever and slightly fewer febrile animals. Because bis(5-amidino-2-benzimidazolyl)methane had no effect on phospholipase A2, the enzyme activity associated with penetration-induced cell injury, it is likely that a trypsin-like protease also plays an essential role either in the physiology of R. rickettsii or as its pathogenic mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J., Geratz J. D., Shaver S. R., Tidwell R. R. Inhibition of respiratory syncytial virus-host cell interactions by mono- and diamidines. Antimicrob Agents Chemother. 1981 Apr;19(4):649–656. doi: 10.1128/aac.19.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J., Geratz J. D., Tidwell R. R. Enhancement of respiratory syncytial virus-induced cytopathology by trypsin, thrombin, and plasmin. Infect Immun. 1983 Apr;40(1):351–358. doi: 10.1128/iai.40.1.351-358.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J., Geratz J. D., Tidwell R. R. Inhibition of respiratory syncytial virus by bis(5-amidino-2-benzimidazolyl)methane. Virology. 1980 Jun;103(2):502–509. doi: 10.1016/0042-6822(80)90207-x. [DOI] [PubMed] [Google Scholar]
- Geratz J. D., Stevens F. M., Polakoski K. L., Parrish R. F., Tidwell R. R. Amidino-substituted aromatic heterocycles as probes of the specificity pocket of trypsin-like proteases. Arch Biochem Biophys. 1979 Oct 15;197(2):551–559. doi: 10.1016/0003-9861(79)90279-0. [DOI] [PubMed] [Google Scholar]
- Hattwick M. A., Retailliau H., O'Brien R. J., Slutzker M., Fontaine R. E., Hanson B. Fatal Rocky Mountain spotted fever. JAMA. 1978 Sep 29;240(14):1499–1503. [PubMed] [Google Scholar]
- Kunze H., Bohn E., Damerau B. Effects of the anti-inflammatory serine esterase inhibitor, FOY, on phospholipase A2 (EC 3.1.1.4) activity in rabbit polymorphonuclear leukocytes. Pharmacol Res Commun. 1983 Oct;15(9):869–878. doi: 10.1016/s0031-6989(83)80094-0. [DOI] [PubMed] [Google Scholar]
- Tidwell R. R., Geratz J. D., Dann O., Volz G., Zeh D., Loewe H. Diarylamidine derivatives with one or both of the aryl moieties consisting of an indole or indole-like ring. Inhibitors of arginine-specific esteroproteases. J Med Chem. 1978 Jul;21(7):613–623. doi: 10.1021/jm00205a005. [DOI] [PubMed] [Google Scholar]
- Tidwell R. R., Geratz J. D., Dubovi E. J. Aromatic amidines: comparison of their ability to block respiratory syncytial virus induced cell fusion and to inhibit plasmin, urokinase, thrombin, and trypsin. J Med Chem. 1983 Feb;26(2):294–298. doi: 10.1021/jm00356a036. [DOI] [PubMed] [Google Scholar]
- Vigo C., Lewis G. P., Piper P. J. Mechanisms of inhibition of phospholipase A2. Biochem Pharmacol. 1980 Feb 15;29(4):623–627. doi: 10.1016/0006-2952(80)90386-x. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Cain B. G. The rickettsial plaque. Evidence for direct cytopathic effect of Rickettsia rickettsii. Lab Invest. 1980 Oct;43(4):388–396. [PubMed] [Google Scholar]
- Walker D. H., Firth W. T., Ballard J. G., Hegarty B. C. Role of phospholipase-associated penetration mechanism in cell injury by Rickettsia rickettsii. Infect Immun. 1983 May;40(2):840–842. doi: 10.1128/iai.40.2.840-842.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Firth W. T., Edgell C. J. Human endothelial cell culture plaques induced by Rickettsia rickettsii. Infect Immun. 1982 Jul;37(1):301–306. doi: 10.1128/iai.37.1.301-306.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Gay R. M., Valdes-Dapena M. The occurrence of eschars in Rocky Mountain spotted fever. J Am Acad Dermatol. 1981 May;4(5):571–576. doi: 10.1016/s0190-9622(81)70059-8. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Mattern W. D. Acute renal failure in Rocky Mountain spotted fever. Arch Intern Med. 1979 Apr;139(4):443–448. [PubMed] [Google Scholar]
- Wike D. A., Burgdorfer W. Plaque formation in tissue cultures by Rickettsia rickettsi isolated directly from whole blood and tick hemolymph. Infect Immun. 1972 Nov;6(5):736–738. doi: 10.1128/iai.6.5.736-738.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike D. A., Ormsbee R. A., Tallent G., Peacock M. G. Effects of various suspending media on plaque formation by rickettsiae in tissue culture. Infect Immun. 1972 Oct;6(4):550–556. doi: 10.1128/iai.6.4.550-556.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike D. A., Tallent G., Peacock M. G., Ormsbee R. A. Studies of the rickettsial plaque assay technique. Infect Immun. 1972 May;5(5):715–722. doi: 10.1128/iai.5.5.715-722.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Miller E. T. Phospholipase A and the interaction of Rickettsia prowazekii and mouse fibroblasts (L-929 cells). Infect Immun. 1982 Oct;38(1):109–113. doi: 10.1128/iai.38.1.109-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch H. Intracellular phospholipases A. Biochim Biophys Acta. 1980 Sep 30;604(2):191–246. doi: 10.1016/0005-2736(80)90574-x. [DOI] [PubMed] [Google Scholar]


