Abstract
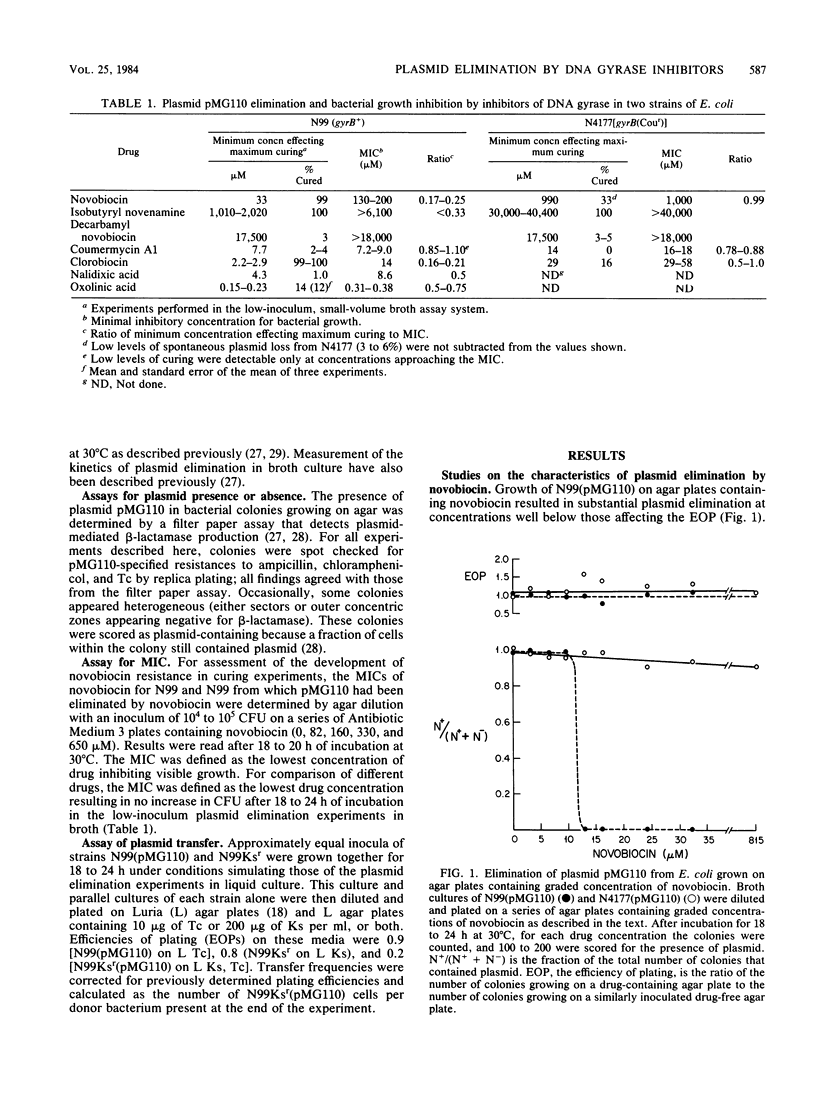
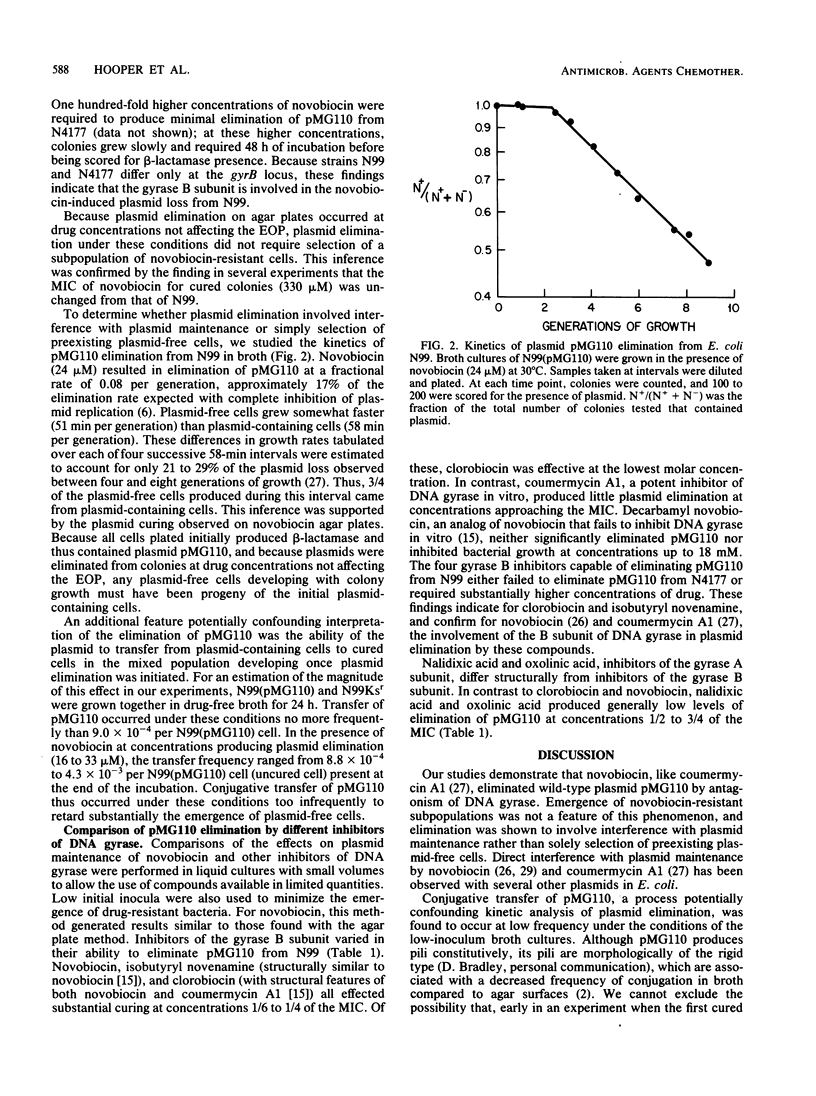
The ability of novobiocin to eliminate (cure) the wild-type plasmid pMG110 from Escherichia coli has been compared with that of other inhibitors of the gyrase B subunit and of the gyrase A subunit. Novobiocin eliminated pMG110 , producing over 99% plasmid loss at concentrations two- to eightfold below the MIC for bacterial growth. Structurally related compounds ( clorobiocin , coumermycin A1, isobutyryl novenamine , and decarbamyl novobiocin) varied in their ability to eliminate pMG110 . Higher concentrations of drugs were required to eliminate pMG110 from a gyrB( Cour ) strain, implicating DNA gyrase in the curing phenomenon. For these drugs, the ratio of the concentration effecting maximal plasmid elimination to the MIC varied from 0.16 to 1.1, indicating that curing cannot be explained simply by inhibition of a pool of DNA gyrase equally available for replication of the bacterial chromosome and the plasmid DNA molecule. Inhibitors of the gyrase A subunit, nalidixic acid and oxolinic acid, eliminated pMG110 only to variable low levels. The differences in the ability of the gyrase A and B subunit antagonists to eliminate plasmids are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M. Mating aggregates in Escherichia coli conjugation. J Bacteriol. 1975 Aug;123(2):505–515. doi: 10.1128/jb.123.2.505-515.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E., Taylor D. E., Cohen D. R. Specification of surface mating systems among conjugative drug resistance plasmids in Escherichia coli K-12. J Bacteriol. 1980 Sep;143(3):1466–1470. doi: 10.1128/jb.143.3.1466-1470.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejka K., Holubová I., Hubácek J. Curing effect of clorobiocin on Escherichia coli plasmids. Mol Gen Genet. 1982;186(1):153–155. doi: 10.1007/BF00422928. [DOI] [PubMed] [Google Scholar]
- Danilevskaya O. N., Gragerov A. I. Curing of Escherichia coli K12 plasmids by coumermycin. Mol Gen Genet. 1980 Apr;178(1):233–235. doi: 10.1007/BF00267235. [DOI] [PubMed] [Google Scholar]
- Drlica K., Snyder M. Superhelical Escherichia coli DNA: relaxation by coumermycin. J Mol Biol. 1978 Apr 5;120(2):145–154. doi: 10.1016/0022-2836(78)90061-x. [DOI] [PubMed] [Google Scholar]
- Durkacz B. W., Sherratt D. J. Segregation kinetics of colicinogenic factor col E1 from a bacterial population temperature sensitive for DNA polymerase I. Mol Gen Genet. 1973;121(1):71–75. doi: 10.1007/BF00353694. [DOI] [PubMed] [Google Scholar]
- Fairweather N. F., Orr E., Holland I. B. Inhibition of deoxyribonucleic acid gyrase: effects on nucleic acid synthesis and cell division in Escherichia coli K-12. J Bacteriol. 1980 Apr;142(1):153–161. doi: 10.1128/jb.142.1.153-161.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. DNA synthesis inhibition and the induction of protein X in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):459–477. doi: 10.1016/0022-2836(76)90240-0. [DOI] [PubMed] [Google Scholar]
- Hahn F. E., Ciak J. Elimination of resistance determinants from R-factor R1 by intercalative compounds. Antimicrob Agents Chemother. 1976 Jan;9(1):77–80. doi: 10.1128/aac.9.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., McHugh G. L., Winters M. B., Swartz M. N. Effects of novobiocin, coumermycin A1, clorobiocin, and their analogs on Escherichia coli DNA gyrase and bacterial growth. Antimicrob Agents Chemother. 1982 Oct;22(4):662–671. doi: 10.1128/aac.22.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer K. N., Cozzarelli N. R. Escherichia coli mutants thermosensitive for deoxyribonucleic acid gyrase subunit A: effects on deoxyribonucleic acid replication, transcription, and bacteriophage growth. J Bacteriol. 1979 Nov;140(2):424–435. doi: 10.1128/jb.140.2.424-435.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh G. L., Swartz M. N. Elimination of plasmids from several bacterial species by novobiocin. Antimicrob Agents Chemother. 1977 Sep;12(3):423–426. doi: 10.1128/aac.12.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K., O'Dea M. H., Gellert M. DNA gyrase: subunit structure and ATPase activity of the purified enzyme. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5960–5963. doi: 10.1073/pnas.75.12.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Pardee A. B. Inhibition of Escherichia coli division by protein X. J Bacteriol. 1978 Mar;133(3):1492–1500. doi: 10.1128/jb.133.3.1492-1500.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Drlica K. DNA gyrase on the bacterial chromosome: DNA cleavage induced by oxolinic acid. J Mol Biol. 1979 Jun 25;131(2):287–302. doi: 10.1016/0022-2836(79)90077-9. [DOI] [PubMed] [Google Scholar]
- Sugino A., Higgins N. P., Brown P. O., Peebles C. L., Cozzarelli N. R. Energy coupling in DNA gyrase and the mechanism of action of novobiocin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4838–4842. doi: 10.1073/pnas.75.10.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Levine J. G. Characterization of a plasmid mutation affecting maintenance, transfer and elimination by novobiocin. Mol Gen Genet. 1979 Jul 13;174(2):127–133. doi: 10.1007/BF00268350. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C., Swartz M. N., McHugh G. L. Antagonism of the B subunit of DNA gyrase eliminates plasmids pBR322 and pMG110 from Escherichia coli. J Bacteriol. 1982 Oct;152(1):338–344. doi: 10.1128/jb.152.1.338-344.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C., Swartz M. N., Swartz M. D., McHugh G. L. Novobiocin-induced elimination of F'lac and mini-F plasmids from Escherichia coli. J Bacteriol. 1983 Dec;156(3):1165–1170. doi: 10.1128/jb.156.3.1165-1170.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C., Swartz M. N., Swartz M. D., McHugh G. L. Rapid method for screening large numbers of Escherichia coli colonies for production of plasmid-mediated beta-lactamases. Antimicrob Agents Chemother. 1983 Feb;23(2):308–312. doi: 10.1128/aac.23.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]


