Abstract
Background
E‐cadherin methylation is important in gastric carcinogenesis. Reversing hypermethylation may halt the carcinogenic process. We have previously reported that Helicobacter pylori infection is associated with E‐cadherin methylation in chronic gastritis patients.
Aim
To examine if eradication of H pylori could reverse E‐cadherin methylation.
Methods
Patients with dyspepsia and positive for H pylori infection, with a mucosal biopsy showing chronic active gastritis, were randomised to receive H pylori eradication therapy (group 1, n = 41) or no treatment (group 2, n = 40), and were followed up prospectively. Gastric mucosae were taken for methylation assay at week 0 (before treatment) and week 6 (after treatment). Archived specimens of intestinal metaplasia with H pylori infection (n = 22) and without (n = 19) were retrieved for methylation analysis. Methylation was assessed using methylation specific polymerase chain reaction and sequencing.
Results
Methylation at E‐cadherin was detected in 46% (19/41) and 17% (7/41) of patients at weeks 0 and 6, respectively, in group 1 (p = 0.004); 78.9% (15/19) of specimens were unmethylated after eradication of H pylori. Mucosal biopsy showed chronic inactive gastritis in 35 patients, intestinal metaplasia in one, and normal mucosa in five at week 6. Methylation was detected in 47.5% (19/40) and 52.5% (21/40) of patients at weeks 0 and 6, respectively, in group 2 (P = 0.5). Gastric mucosal biopsy showed persistent chronic active gastritis in all cases. Methylation frequency did not differ in H pylori positive or negative intestinal metaplastic specimens (72.7% v 63%; p = 0.5).
Conclusion
H pylori eradication therapy could reverse methylation in patients with chronic gastritis. This demonstrates an environmental effect on methylation.
Keywords: E‐cadherin methylation, gastric cancer, intestinal metaplasia, Helicobacter pylori
Gastric carcinogenesis is a multistep process involving multiple genetic and epigenetic events, with a postulated intestinal metaplasia‐dysplasia‐invasive carcinoma sequence during morphologic progression.1Helicobacter pylori infection is an important aetiological risk factor in gastric cancer, and has been classified as a group I or definite carcinogen by the World Health Organisation's International Agency for Research on Cancer.2
E‐cadherin is an adhesion molecule that is expressed on all epithelial cells.3,4 It is an important tumour suppressor and invasion suppressor gene. In gastric carcinogenesis, the critical role of E‐cadherin is underlined by the observation that familial gastric cancer is related to germline mutations of the E‐cadherin gene.5,6 Furthermore, somatic mutations of E‐cadherin were found in approximately 50% of gastric carcinomas of the diffuse histological type.7 Finally, in two kindreds with familial gastric cancer and germline E‐cadherin mutation, promoter CpG hypermethylation was found to be the second “genetic hit” in abrogating E‐cadherin expression.8 It is now increasingly recognised that epigenetic silencing of gene expression by promoter CpG hypermethylation is an important alternative mechanism in inactivating tumour suppressor genes and tumour associated genes in cancers,9,10 and in gastric cancer and its precursors.11,12
We have previously shown that expression of E‐cadherin protein was decreased early in precancerous lesions of gastric cancer, suggesting E‐cadherin may play an early role in gastric cancer.13 Methylation of E‐cadherin was identified early in precancerous lesions of gastric cancer, being found in 31% of gastric mucosa with chronic gastritis.14 More importantly, H pylori was an independent risk factor associated with methylation of E‐cadherin in non‐lesional gastric mucosa from patients with dyspepsia.14
Targeting epigenetic changes that occur before the development of frank malignancy as chemopreventive intervention offers the maximal impact.10,15 Hence in gastric cancer, early reversal of promoter methylation at precanerous lesions before the development of frank gastric cancer might halt gastric carcinogenesis. We hypothesised that H pylori infection may be an important aetiology in causing methylation at E‐cadherin and that eradication of H pylori might reverse methylation at E‐cadherin. We tested this hypothesis by examining methylation of the E‐cadherin gene in gastric mucosa with chronic gastritis from patients without gastric cancer before and after eradication of H pylori, and also in intestinal metaplasia from patients with and without H pylori infection.
Materials and methods
Patients and specimens
Two cohorts of patients were studied. The first cohort, which constituted the majority of patients in this study, was derived from a prospective randomised controlled study. Ninety patients from the Department of Medicine, Queen Mary Hospital, Hong Kong, who had upper endoscopy for investigation of dyspepsia and were confirmed to be H pylori positive were recruited to the study. The presence of H pylori infection was confirmed by rapid urease test and subsequent histological analysis using both haematoxylin‐eosin and the modified Giemsa stains. Equivocal cases were excluded from the analysis. This approach has been validated previously in our centre.16 Antral gastric mucosal biopsies were taken for histological analysis, E‐cadherin immunohistochemical staining, and methylation study (week 0). Haematoxylin‐eosin stained slides were evaluated for the presence of chronic gastritis, intestinal metaplasia, or dysplasia. Histological assessment was categorised according to the Sydney classification.
Patients were included in the study only if the gastric mucosal histology showed chronic active gastritis after confirmation of H pylori infection. Patients were then randomised into two groups: group 1 subjects received H pylori eradication therapy while group 2 received no treatment. H pylori eradication therapy consisted of one week of amoxicillin 1000 mg twice/day, clarithromycin 500 mg twice/day, and omeprazole 20 mg twice/day. All patients were followed up prospectively for six weeks. Upper endoscopy was repeated again at the end of six weeks (week 6). The status of H pylori infection was reassessed using the same method. Antral gastric mucosal biopsies were again taken for histology, immunostaining, and methylation analysis.
In order to assess the degree of concordant methylation at the antrum, five additional random antral mucosa biopsies were taken from each of the 10 patients at week 0. Concordant methylation was defined as the same methylation status (either negative or positive) at the E‐cadherin gene in at least four biopsy samples in each patient. Informed consent for tissue procurement was obtained from all patients.
The second cohort comprised archival antral biopsy specimens with documented intestinal metaplasia from patients who were H pylori positive (n = 22) and from those who were H pylori negative (n = 19) as controls. The study was approved by our institutional review board.
Methylation specific polymerase chain reaction (MSP)
Gastric mucosal tissue was obtained by microdissection from 5 μm thick haematoxylin‐ eosin stained, paraffin embedded tissue sections without a coverslip, followed by DNA extraction, as described previously.17 The methylation status of the E‐cadherin promoter was determined by bisulfite treatment of DNA followed by MSP, as described previously.18 MSP was performed by an operator unaware of the clinical data of the specimens. Briefly, 2 μg of DNA were denatured with 2 M NaOH at 37°C for 10 minutes, followed by incubation with 3 M sodium bisulfite, pH 5.0, at 50°C for 16 hours. Bisulfite treated DNA was then purified (DNA Cleanup Kit; Promega, Madison, Wisconsin, USA), incubated with 3 M NaOH at room temperature for five minutes, precipitated with 10 M ammonium acetate and 100% ethanol, washed with 70% ethanol, and resuspended in 20 μl of distilled water. DNA (2 μm) was then amplified for the E‐cadherin gene by polymerase chain reaction (PCR) with the primers described by Herman and colleagues (table 1).18 CpGenome Universal Methylated DNA (Intergen, Purchase, New York, USA) and reagent blanks were used as positive and negative controls in each experiment. All tests were performed in duplicate. For confirmation of the specificity of the MSP, PCR products from the methylated and unmethylated primers were gel purified and sequenced, as previously described.19
Table 1 Primers and polymerase chain reaction conditions for E‐cadherin, DAPK, MGMT, hMLH1, p16, and OR beta.
| Locus | Allele | Sense primers | Antisense primers | Annealing temp (°C) |
|---|---|---|---|---|
| E‐cadherin | Methylated | 5′′‐ TTAGGTTAGAGGGTTATCGCGT ‐3′ | 5′‐ TAACTAAAAATTCACCTACCGAC‐3′ | 57 |
| Unmethylated | 5′‐ TAATTTTAGGTTAGAGGGTTATTGT‐3′ | 5′‐ CACAACCAATCAACAACACA‐3′ | 53 | |
| DAPK | Methylated | 5′‐ GGATAGTCGGATCGAGTTAACGTC ‐3′ | 5′‐ CCCTCCCAAACGCCGA ‐3′ | 60 |
| Unmethylated | 5′‐GGAGGATAGTTGGATTGAGTTAATGTT ‐3′ | 5′‐ CAAATCCCTCCCAAACACCAA ‐3′ | 60 | |
| MGMT | Methylated | 5′‐ TTTCGACGTTCGTAGGTTTTCGC ‐3′ | 5′‐ GCACTCTTCCGAAAACGAAACG ‐3′ | 59 |
| Unmethylated | 5′‐TTTGTGTTTTGATGTTTGTAGGTTTTTGT‐3′ | 5′‐AACTCCACACTCTTCCAAAAACAAAACA‐3′ | 59 | |
| hMLH1 | Methylated | 5′‐ TATATCGTTCGTAGTATTCGTGT ‐3′ | 5′‐ TCCGACCCGAATAAACCCAA‐3′ | 60 |
| Unmethylated | 5′‐ TTTTGATGTAGATGTTTTATTAGGGTTGT ‐3′ | 5′‐ ACCACCTCATCATAACTACCCACA ‐3′ | 60 | |
| p16 | Methylated | 5′‐TTATTAGAGGGTGGGGCGGATCGC‐3′ | 5′‐GACCCCGAACCGCGACCGTAA‐3′ | 65 |
| Unmethylated | 5′‐TTATTAGAGGGTGGGGTGGATTGT‐3′ | 5′‐CAACCCCAAACCACAACCATAA‐3′ | 60 | |
| OR beta | Methylated | 5′‐TTTGGAAGGTGGGTTTGGTC‐3′ | 5′‐CGCATACAAATATAATAACTAACG‐3′ | 49 |
| Unmethylated | 5′‐TTTGGAAGGTGGGTTTGGTT‐3′ | 5′‐CACATACAAATATAATAACTAACA‐3′ | 45 |
DAPK, DAP kinase; MGMT, O6‐methyl‐guanine methyltransferase; hMLH1, human Mut L homologue; OR beta, oestrogen receptor beta.
Pre‐ and post‐H pylori eradication methylation pattern was also assessed in 10 additional pairs of biopsy specimens from group 1 at DAP kinase (DAPK), O6‐methyl‐guanine methyltransferase (MGMT), human Mut L homologue (hMLH1), p16 genes,20 and oestrogen receptor beta (table 1).21
Immunohistochemical staining for E‐cadherin
E‐cadherin expression was examined by immunostaining using the avidin‐biotin complex immunoperoxidase method, as described previously.13 Briefly, 4 μm thick tissue slides were deparaffinised in xylene and rehydrated serially with alcohol and water. Endogenous peroxidase was blocked with 3% hydrogen peroxide for 10 minutes, followed by microwave antigen retrieval for nine minutes at 95°C in 10 mM sodium citrate buffer, pH 6.0. Slides were then incubated with an avidin conjugated monoclonal anti‐E‐cadherin antibody (HECD‐1, 1:500 dilution in phosphate buffered saline; Zymed Laboratories Inc., South San Francisco, USA) in a moist chamber at 37°C for one hour. Bound antibody was detected by a biotinylated secondary antibody and the avidin‐biotin complex immunoperoxidase method (Dako Corp., Carpinteria, California, USA). Slides were finally counterstained with Mayer's haematoxylin. As a negative control, the primary antibody was replaced with mouse IgG. Slides with normal colonic mucosa were used as positive controls. E‐cadherin staining was classified as altered when cytoplasmic and/or membranous staining was reduced or absent.
Statistical analysis
The χ2 test was used to compare categorical associations and the Student's t test for continuous associations. Two sided tests were used to calculate p values.
Results
Demographic data from patients who presented with dyspepsia
Two patients from the first cohort were excluded from the study because the initial gastric mucosal biopsies showed evidence of intestinal metaplasia. The remaining 88 patients were randomised into groups 1 and 2. Three patients from group 1 and four patients from group 2 defaulted for the second upper endoscopy and were excluded from the final analysis. There were nine men and 32 women in group 1 and 11 men and 29 women in group 2 (p = 0.6). Mean age of group 1 patients was 51 (11) years and 47 (16) years in group 2 (p = 0.2).
Promoter methylation at E‐cadherin at week 0 and week 6 in group 1 patients
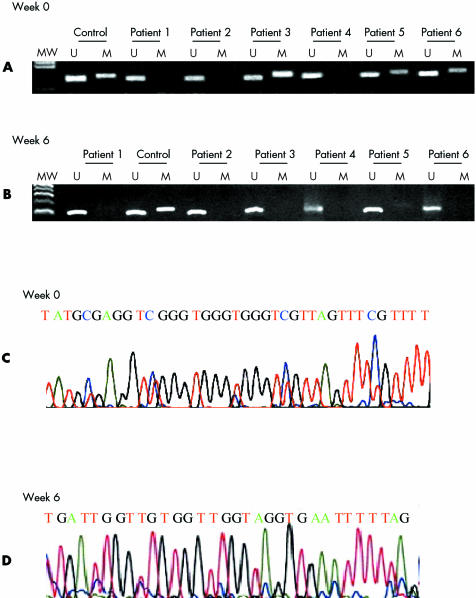
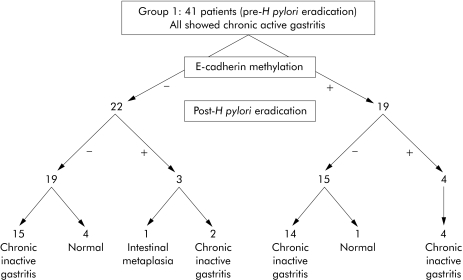
At week 0, chronic active gastritis was present in all specimens. Promoter methylation at E‐cadherin was present in 46% (19/41) of the gastric mucosa specimens (examples in fig 1A). There was no difference in mean age between patients who had methylation in their gastric mucosa (51 (9) years) and those who had not (51 (11) years) (p = 0.8). At week 6 after H pylori eradication therapy, none of the patients showed persistent H pylori infection. Normal histology was observed in the antral gastric biopsy specimens from five patients, while 35 biopsy specimens showed chronic inactive gastritis, and one specimen showed intestinal metaplasia (fig 2). Promoter methylation at E‐cadherin was only present in 17% (7/41) of the gastric mucosa specimens, which was significantly different from that in the pre‐eradication specimens (p = 0.004) (table 2; examples in fig 1B)—that is, among the 19 specimens which were positively methylated before H pylori eradication, 15 (78.9%) became unmethylated after eradication therapy (fig 2). The disappearance of promoter methylation at E‐cadherin was associated with reversal of chronic active gastritis to inactive gastritis in 14 patients, and to normal mucosa in one patient. There were three unmethylated specimens which became methylated after eradication therapy (fig 2). One showed a histological change from chronic active gastritis to intestinal metaplasia (fig 2). Specificity of E‐cadherin methylation was confirmed by DNA sequencing (fig 1C, 1D). Patients with or without E‐cadherin methylation after H pylori eradication did not differ in mean age (50 (8) v 52 (11) years; p = 0.5).
Figure 1 CpG island methylation pattern at the E‐cadherin gene in gastric mucosa from patients with dyspepsia. MW, molecular weight marker; U, unmethylated band; M, methylated band. (A) At week 0 before eradication of Helicobacter pylori, methylation was present in patient Nos 3, 5, and 6. (B) At week 6 after eradication of H pylori, methylation was not present in any patient. (C) At week 0 before eradication of H pylori, the methylated product was confirmed by sequencing using the same methylated primer. The unmethylated cytosines were converted to thymidine (red colour) while the methylated cytosines remained unchanged (blue colour). (D) At week 6 after eradication of H pylori, the methylated product was again confirmed by sequencing using the same methylated primer. No methylated cytosine was seen.
Figure 2 Summary of the methylation and histology status of the 41 patients in group 1, before and after Helicobacter pylori eradication.
Table 2 E‐cadherin methylation in gastric mucosal specimens before and after Helicobacter pylori eradication.
| E‐cadherin methylated | E‐cadherin unmethylated | |
|---|---|---|
| Before H pylori eradication | 19 | 22 |
| After H pylori eradication | 7* | 34 |
*E‐cadherin was previously methylated in four patients but unmethylated in three patients.
Promoter methylation at E‐cadherin at week 0 and week 6 in group 2 patients
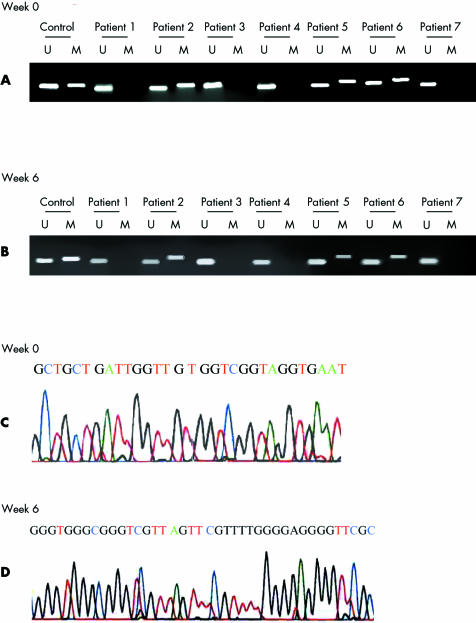
At week 0, chronic active gastritis was present in all specimens. Promoter methylation at E‐cadherin was present in 47.5% (19/40) of the gastric mucosa specimens (examples in fig 3A). Mean age of patients with positive and negative methylation in their gastric mucosa was 53 (10) years and 43 (14) years, respectively (p = 0.05). At week 6, none of the specimens showed inactive gastritis or normal gastric epithelium. Promoter methylation at E‐cadherin was observed in 52.5% (21/40) of specimens (examples in fig 3B) which showed no difference in methylation frequency compared with week 0 (p = 0.5). The methylation status at E‐cadherin at week 0 and week 6 was confirmed by sequencing (fig 3C, 3D). Three specimens with negative methylation at week 0 became positive at week 6. One specimen with positive methylation at week 0 became negative at week 6.
Figure 3 CpG island methylation at the E‐cadherin gene in gastric mucosa from patients with dyspepsia without receiving Helicobacter pylori eradication therapy. MW, molecular weight marker; U, unmethylated band; M, methylated band. (A) At week 0, methylation was present in patient Nos 2, 5, and 6. (B) At week 6, methylation was still present in patient Nos 2, 5, and 6. C. At week 0, methylated products were confirmed by sequencing using the same methylated primer. Methylated cytosines were seen. (D) At week 6, methylated cytosines were still observed after sequencing using the same methylated primer.
Promoter methylation at other genes at week 0 and week 6 in 10 of the group 1 patients
The methylation pattern at week 0 and week 6 at these genes are summarised in table 3. Disappearance of methylation was not observed in these genes.
Table 3 Promoter methylation at DAPK, MGMT, hMLH1, OR beta, and p16 at week 0 and week 6 in 10 of the group 1 patients.
| Week 0 (n = 10) (No (%)) | Week 6 (n = 10) (No (%)) | |
|---|---|---|
| DAPK | 2 (20) | 1 (10) |
| MGMT | 0 | 2 (20) |
| hMLH1 | 1 (10) | 1 (10) |
| OR beta | 6 (60) | 5 (50) |
| p16 | 0 | 0 |
| E‐cadherin | 5 (50) | 2 (20) |
DAPK, DAP kinase; MGMT, O6‐methyl‐guanine methyltransferase; hMLH1, human Mut L homologue; OR beta, oestrogen receptor beta.
Analysis for the presence of concordant methylation
Each of the 10 patients had five additional antral biopsies taken. Eight showed concordant methylation: four showed concordant positive E‐cadherin methylation (at least 4/5 biopsies were positive for E‐cadherin methylation from each of these patients) and four showed concordant negative methylation (all five biopsies were negative for E‐cadherin methylation from each of these patients).
Immunostaining of E‐cadherin
Immunostaining of gastric mucosae from group 1 and group 2 patients showed strong membranous staining in the gastric epithelium, and there was no difference in staining between pre‐ and post‐H pylori eradication in group 1 patients.
Promoter methylation at E‐cadherin in the intestinal metaplasia specimens
In the second cohort of patients, among 19 H pylori negative specimens, 63% (12) showed E‐cadherin methylation. Among the 22 H pylori positive specimens, 72.7% (16) of specimens were methylated (p = 0.5).
Discussion
Our study showed that by eradicating H pylori infection early in the stage of chronic gastritis in patients without gastric cancer, promoter methylation at E‐cadherin disappeared in a high proportion of patients.
The importance of the E‐cadherin gene in gastric carcinogenesis has been well demonstrated,5,6,7 and hence the methylation pattern at E‐cadherin before and after eradication of H pylori was of particular interest in our study. We have also studied other genes which have been reported to be methylated in patients with chronic gastritis.20 The genes that we chose are involved in cell cycle regulation (p16), DNA repair or protection (hMLH1, MGMT), and apoptosis (DAP kinase).
In addition to the important role E‐cadherin plays in gastric cancer, we have also demonstrated in our previous work that methylation of the E‐cadherin promoter is associated with gastric cancer and have generated a hypothesis linking E‐cadherin methylation, H pylori infection, intestinal metaplasia, and gastric carcinogenesis.13,14 The current study again showed that H pylori infection was associated with E‐cadherin methylation at the gastric mucosa in dyspeptic subjects without gastric cancer. Despite the fact that the role of E‐cadherin methylation in the gastric epithelium in these dyspeptic subjects in the future development of gastric cancer remains uncertain, targeting epigenetic changes that occur before the development of frank malignancy as chemopreventive intervention may offer the maximal impact. Hence the disappearance of E‐cadherin methylation in these dyspeptic patients may be important for preventing future development of gastric cancer.
In the current study we observed that the disappearance of E‐cadherin methylation after H pylori eradication was associated with a decrease in activity of chronic gastritis, according to the Sydney classification. The underlying mechanism of the reversal of the methylation process is still uncertain. We postulate that this may relate to the decrease in inflammation after H pylori eradication. It is well known that promoter methylation is associated with chronic inflammatory conditions, such as inflammatory bowel disease,22,23 oesophageal mucosa in patients with Barrett's oesophagitis,24,25 and in liver tissues in chronic hepatitis.26 We also observed in the current study that, in the presence of intestinal metaplasia, E‐cadherin methylation did not associate with the presence of H pylori infection. This supports the fact that the presence of E‐cadherin methylation relates to the underlying chronic inflammatory condition. In contrast, it has been reported that methylation dependent gene silencing can be induced by interleukin 1β via the action of nitric oxide.27 It has also been recently reported that H pylori upregulates mRNA expression, promoter activity, and enzyme activity of inducible spermine oxidase in human gastric epithelial cells, resulting in DNA damage and apoptosis.28 Thus the disappearance of methylation at the stage of chronic gastritis may also be directly related to abolishment of oxidative stress caused by H pylori. The current observation is intriguing and will require further extensive studies to confirm the underlying mechanism.
In addition, we also assessed the presence of concordant methylation within the antrum from 10 patients. We found 80% concordant methylation status. This implies that the possibility that the difference in E‐cadherin methylation at week 0 and week 6 was due to random biopsy errors is less likely.
Despite the fact that we observed differences in E‐cadherin methylation pre‐ and post‐H pylori eradication in gastric mucosae, we did not observe any difference in immunostaining at E‐cadherin in these specimens. We postulate that this may be due to the fact that MSP is a very sensitive method for detecting methylation. The sensitivity of MSP was 10−3 (results not shown). On the other hand, immunohistochemical staining is a qualitative method, and is not as sensitive as PCR in detecting subpopulations of cells with gene methylation and hence downregulation of E‐cadherin. A large number of cells may be needed to be methylated before this is reflected in expression by immunostaining.
Methylation analysis at other genes was also performed in 10 pairs of pre‐ and post‐H pylori eradicated specimens. However, no difference in methylation at these genes was observed. This could be due to the small sample size. On the other hand, methylation frequencies in gastric mucosa at DAPK, MGMT, hMLH1, and p16 genes were 41%, 18.7%, 10.9%, and 4.1%, respectively, according to Kang and colleagues,20 which was not as high as that observed in E‐cadherin, and this may also account for the difference.
The current study corroborates the observation in a prospective randomised controlled study from our group showing that eradicating H pylori prevented gastric cancer in patients with chronic gastritis but not in those with existing precursor lesions, such as intestinal metaplasia.29 The current study may partially explain the clinical observation. Whether disappearance of E‐cadherin methylation at the gastric mucosa in dyspeptic patients can eventually prevent gastric cancer development still requires further investigation.
Acknowledgements
This study was supported by the Michael Kadoorie Cancer Genetics Research Project of the Kadoorie Charitable Foundation, Hong Kong, and the Gordon YH Chiu Stomach Cancer Research Fund, University of Hong Kong, Hong Kong.
Abbreviations
PCR - polymerase chain reaction
MSP - methylation specific polymerase chain reaction
Footnotes
Conflict of interest: None declared.
The abstract was presented orally at the AGA Distinguished Abstract Plenary Session, GI Oncology Plenary Session: Frontiers of Clinical Medicine and Translational Research, Digestive Disease Week, New Orleans, USA, May 2004.
References
- 1.Correa P. A human model of gastric carcinogenesis. Cancer Res 1988483554–3560. [PubMed] [Google Scholar]
- 2. IARC working group on the evaluation of carcinogenic risks to humans, schistosomes, liver flukes, helicobacter pylori. IARC monographs on the evaluation of carcinogenic risks to humans, vol 61. Lyon: International Agency for Research on Cancer, 19941–241. [PMC free article] [PubMed]
- 3.Takeichi M. Cadherins: a molecular family important in selective cell‐cell adhesion. Annu Rev Biochem 199059237–252. [DOI] [PubMed] [Google Scholar]
- 4.Grunwald G. The structural and functional analysis of cadherin calcium‐dependent cell adhesion molecules. Curr Opin Cell Biol 19935797–805. [DOI] [PubMed] [Google Scholar]
- 5.Guilford P, Hopkins J, Harraway J.et al E‐cadherin germline mutations in familial gastric cancer. Nature 1998392402–405. [DOI] [PubMed] [Google Scholar]
- 6.Gayther S A, Gorringe K L, Ramus S J.et al Identification of germ‐line E‐cadherin mutations in gastric cancer families of European origin. Cancer Res 1998584086–4089. [PubMed] [Google Scholar]
- 7.Becker K F, Atkinson M J, Reich U.et al E‐cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res 1994543845–3852. [PubMed] [Google Scholar]
- 8.Grady W M, Willis J, Guilford P J.et al Methylation of the CDH1 promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet 20002616–17. [DOI] [PubMed] [Google Scholar]
- 9.Jones P A, Buckley J D. The role of DNA methylation in cancer. Adv Cancer Res 1990541–23. [DOI] [PubMed] [Google Scholar]
- 10.Herman J G, Baylin S B. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med 2003492042–2054. [DOI] [PubMed] [Google Scholar]
- 11.Tamura G, Yin J, Wang S.et al E‐Cadherin gene promoter hypermethylation in primary human gastric carcinomas. J Natl Cancer Inst 200092569–573. [DOI] [PubMed] [Google Scholar]
- 12.Kang G H, Shim Y H, Jung H Y.et al CpG island methylation in premalignant stages of gastric carcinoma. Cancer Res 200162847–2851. [PubMed] [Google Scholar]
- 13.Chan A O, Wong B C Y, Lan H Y.et al Deregulation of E‐cadherin‐catenin complex in precancerous lesions of gastric adenocarcinomas. J Gastroenterol Hepatol 200318534–539. [DOI] [PubMed] [Google Scholar]
- 14.Chan A O, Lam S K, Wong B C Y.et al Methylation of E‐cadherin gene in gastric mucosa associated with Helicobacter pylori infection and gastric cancer. Gut 200352502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopelovich L, Crowell J A, Fay J R. The epigenome as a target for cancer chemoprevention. J Natl Cancer Inst 2003951747–1757. [DOI] [PubMed] [Google Scholar]
- 16.Wong B C, Wong W M, Wang W H.et al An evaluation of invasive and non‐invasive tests for the diagnosis of Helicobacter pylori infection in Chinese—the best tests for routine clinical use and research purposes. Aliment Pharmacol Ther 200115505–511. [DOI] [PubMed] [Google Scholar]
- 17.Moskaluk C A, Kern S E. Microdissection and polymerase chain reaction amplification of genomic DNA from histological tissue sections. Am J Pathol 19971501547–1552. [PMC free article] [PubMed] [Google Scholar]
- 18.Herman J G, Graff J R, Myohanen S.et al Methylation‐specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A 1996939821–9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chim C S, Liang R, Tam C Y.et al Methylation of p15 and p16 genes in acute promyelocytic leukemia: potential diagnostic and prognostic significance. J Clin Oncol 2000192033–2040. [DOI] [PubMed] [Google Scholar]
- 20.Kang G H, Lee H J, Hwang K S.et al Aberrant CpG island hypermethylation of chronic gastritis, in relation to aging, gender, intestinal metaplasia, and chronic inflammation. Am J Pathol 20031631551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki M, Tanaka Y, Perinchery G.et al Methylation and inactivation of estrogen, progesterone, and androgen receptors in prostate cancer. J Natl Cancer Inst 200294384–390. [DOI] [PubMed] [Google Scholar]
- 22.Sato F, Harpaz N, Shibata D.et al Hypermethylation of the p14 (ARF) gene in ulcerative colitis‐associated colorectal carcinogenesis. Cancer Res 2002621148–1151. [PubMed] [Google Scholar]
- 23.Issa J P, Ahuja N, Toyota M.et al Accelerated age‐related CpG island methylation in ulcerative colitis. Cancer Res 2001613573–3577. [PubMed] [Google Scholar]
- 24.Bian Y S, Osterheld M C, Fontolliet C.et al p16 inactivation by methylation of the CDKN2A promoter occurs early during neoplastic progression in Barrett's esophagus. Gastroenterology 20021221113–1121. [DOI] [PubMed] [Google Scholar]
- 25.Wong D J, Paulson T G, Prevo L J.et al p16(INK4a) lesions are common, early abnormalities that undergo clonal expansion in Barrett's metaplastic epithelium. Cancer Res 2001618284–8289. [PubMed] [Google Scholar]
- 26.Kaneto H, Sasaki S, Yamamoto H.et al Detection of hypermethylation of the p16(INK4A) gene promoter in chronic hepatitis and cirrhosis associated with hepatitis B or C virus. Gut 200148372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hmadcha A, Bedoya F J, Sobrino F.et al Methylation‐dependent gene silencing induced by interleukin 1beta via nitric oxide production. J Exp Med 19991901595–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu H, Chaturvedi R, Cheng Y.et al Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res 2004648521–8525. [DOI] [PubMed] [Google Scholar]
- 29.Wong B C Y, Lam S K, Wong W M, and the China Gastric Cancer Study Group et al Prospective randomized placebo‐controlled study of Helicobacter pylori eradication to prevent gastric cancer in a high‐risk region of China. JAMA 2004291187–194. [DOI] [PubMed] [Google Scholar]