The development of gastric epithelial neoplasia is closely linked to precursor conditions in the background mucosa.1,2,3 Recently, endoscopic mucosal resection (EMR) has become widely used for the treatment of gastric neoplasia,4 resulting in almost complete conservation of the patient's stomach. To determine the risk of a second cancer in the stomach that once gave rise to epithelial neoplasia, we conducted a long term retrospective cohort study of 255 patients with primary gastric epithelial neoplasia who underwent curative resection by EMR between 1983 and 2002 at our hospital.
Characteristics of the subjects at the initial treatment are shown in table 1. Synchronous multiple neoplasias were confirmed in 19 (7.5 %) of the 255 subjects; 56 patients had adenomas and 199 had cancer. In the eight patients with both gastric cancer and adenoma, cancer was taken as the representative histology. In this study, we defined lesions classified as category 3 in the Vienna classification5 as “adenoma” and categories 4 and 5 as “cancer”.
Table 1 Characteristics of the 255 patients at the initial endoscopic treatment and multivariate analysis* as predictors for subsequent gastric cancer.
| Characteristic | Hazard ratio | 95% CI | p Value§ | |
|---|---|---|---|---|
| No of patients | 255 | |||
| Male sex | 183 (71.8%) | 1.11 | 0.52–2.39 | 0.79 |
| Age (years) (mean (SD)) | 67.9 (9.2) | 1.54‡ | 1.07–2.21 | 0.02 |
| Histology | ||||
| Adenoma | 56 | 0.98 | 0.48–2.00 | 0.95 |
| Carcinoma† | 199 | |||
| Synchronous multiplicity | ||||
| Solitary | 236 (92.5%) | |||
| Multiple | 19 (7.5%) | 4.10 | 1.92–8.79 | <0.01 |
| Adenomas | 2 | |||
| Adenoma and carcinoma | 8 | |||
| Carcinomas | 9 | |||
| Surveillance period (months) (mean (SD)) | 51.6 (34.9) | |||
*The Cox proportional hazard model was used.
†Carcinoma was taken as the representative histology of the case when the patient had multiple neoplasias of both carcinoma and adenoma.
‡Increment of 10 years.
§p values were calculated using the Wald test.
A second gastric cancer developed in 43 of the 255 patients. The crude incidence rate of the second gastric cancer was 3.9 per 100 person years. Kaplan‐Meier estimates showed that the three year and six year cumulative incidence rates of newly developed gastric cancers were 10% and 20%, respectively. The cumulative incidence of subsequent cancer increased steadily throughout the observation.
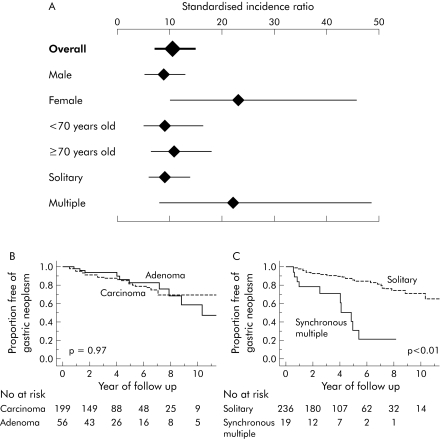
To compare the risk of new development of gastric cancer in our patients with that of general population in Japan,10 the standardised incidence ratio (SIR) was calculated. SIR for newly developed cancer in our patients was 10.4 (95% confidence interval 7.0–14.9). SIRs stratified according to sex, age, and synchronous multiplicity at the initial treatment are also shown in fig 1A.
Figure 1 (A) Standardised incidence ratio (SIR) of newly developed gastric cancers according to sex, age, and multiplicity at the initial treatment. SIRs were calculated based on age, sex, and calendar year specific incidence rates of gastric cancer in the general population of Japan.10 Values are SIRs (95% confidence intervals) calculated based on Poisson distribution. This analysis was restricted to 231 patients who received endoscopic mucosal resection (EMR) and whose subsequent gastric cancer developed before 1999 because data for the estimated incidence rate of gastric cancer in Japan was available only between 1975 and 1999.10 (B, C) Cumulative incidence of subsequent gastric cancer after EMR. The Kaplan‐Meier method was used to calculate the cumulative incidence. The cumulative incidence in patients with gastric adenoma did not differ significantly from the incidence in those with gastric cancer as the initial lesion (p = 0.97) (B). The cumulative incidence of subsequent gastric cancer was significantly higher in patients with synchronous multiple lesions than in those with a single lesion as the initial neoplasia (p<0.01) (C). The log rank test was used to calculate p values.
Furthermore, we analysed the factors predicting subsequent gastric cancer development. Ten of the 56 adenoma and 33 of the 199 cancer patients had newly developed gastric cancer. The crude incidence rates of the second cancer were exactly the same (3.9 per 100 person years) in both groups, and the cumulative incidences did not differ between the two groups using the Kaplan‐Meier method (p = 0.97; fig 1B). Patients with synchronous multiple lesions were at a significantly higher risk than those with a solitary lesion (p<0.01; fig 1C). Multivariate analysis confirmed the above findings (table 1).
All of our patients were positive for Helicobacter pylori but did not receive eradication therapy because many underwent EMR before the mid 1990s. Thus a very high rate of H pylori infection appears to be at least partly responsible for the high incidence of the second cancer in our patients.6 However, the incidence in our patients was much higher than H pylori positive Japanese3,7 and Chinese8 patients without a history of gastric neoplasia. Thus factors other than H pylori infection were also involved in the development of the second gastric cancer in our patients with previous gastric neoplasia.
An important finding of our study was that patients with gastric adenoma had the same risk for the development of a subsequent gastric cancer as those with gastric cancer. In addition, we found that a considerable number of our patients with gastric adenoma (12.5%) had concurrent gastric cancer. Thus although gastric adenoma may rarely progress to cancer,9 it is likely that gastric cancers and adenomas develop in the same background of the gastric mucosa, probably with similar genetic alterations or environmental conditions.
Taken together, our results indicate that patients with a previous history of either gastric adenoma or cancer are at a higher risk of subsequent development of gastric cancer. Once neoplasia develops, the gastric mucosa should be considered to have an exceedingly high potential to develop cancers.
Acknowledgement
We thank Professor T Fujimori of the Department of Surgical and Molecular Pathology, Dokkyo University School of Medicine, Tochigi, Japan, for useful advice on histopathology.
Footnotes
Conflict of interest: None declared.
References
- 1.Fuchs C S, Mayer R J. Gastric carcinoma. N Engl J Med 199533332–41. [DOI] [PubMed] [Google Scholar]
- 2.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992526735–6740. [PubMed] [Google Scholar]
- 3.Uemura N, Okamoto S, Yamamoto S.et al Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001345784–789. [DOI] [PubMed] [Google Scholar]
- 4.Ono H, Kondo H, Gotoda T.et al Endoscopic mucosal resection for treatment of early gastric cancer. Gut 200148225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlemper R J, Riddell R H, Kato Y.et al The Vienna classification of gastrointestinal epithelial neoplasia. Gut 200047251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J Q, Sridhar S, Chen Y.et al Meta‐analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology 19981141169–1179. [DOI] [PubMed] [Google Scholar]
- 7.Ohata H, Kitauchi S, Yoshimura N.et al Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer 2004109138–143. [DOI] [PubMed] [Google Scholar]
- 8.Wong B C, Lam S K, Wong W M.et al Helicobacter pylori eradication to prevent gastric cancer in a high‐risk region of China: a randomized controlled trial. JAMA 2004291187–194. [DOI] [PubMed] [Google Scholar]
- 9.Yamada H, Ikegami M, Shimoda T.et al Long‐term follow‐up study of gastric adenoma/dysplasia. Endoscopy 200436390–396. [DOI] [PubMed] [Google Scholar]
- 10.Ajiki W, Tsukuma H, Oshima A. Cancer incidence and incidence rates in Japan in 1999: estimates based on data from 11 population‐based cancer registries. Jpn J Clin Oncol 200434352–356. [DOI] [PubMed] [Google Scholar]