Abstract
Five RNA- and two DNA-containing viruses were propagated in Vero cells and tested for their ability to replicate in the presence of halothane (2-bromo-2-chloro-1,1,1-trifluoroethane), a commonly used inhalational anesthetic. Halothane did not affect poliovirus replication at any anesthetic concentration tested, but all other viruses were either partially or totally inhibited by clinical doses of the anesthetic. Replication of Sendai virus, simian virus 40, vesicular stomatitis virus, and herpes simplex virus type 1 were moderately inhibited by halothane exposure. At concentrations of 2.2% (vol/vol) halothane, peak virus titers were reduced by ca. 2 orders of magnitude for vesicular stomatitis virus and simian virus 40, 3.5 orders of magnitude for Sendai virus, and 4 orders of magnitude for herpes simplex virus. Newcastle disease virus and measles virus were the most susceptible to exposure to halothane. Total inhibition of the replication of these viruses occurred at 1.6 to 2.0% halothane. All of the viruses whose replication was susceptible to the action of halothane were inhibited in a concentration-dependent manner. Furthermore, with the exception of simian virus 40, the inhibition of the replication of all viruses was reversible after halothane removal, although total recovery of virus synthesis was not observed unless the culture medium was changed or the pH was adjusted after anesthetic removal.
Full text
PDF
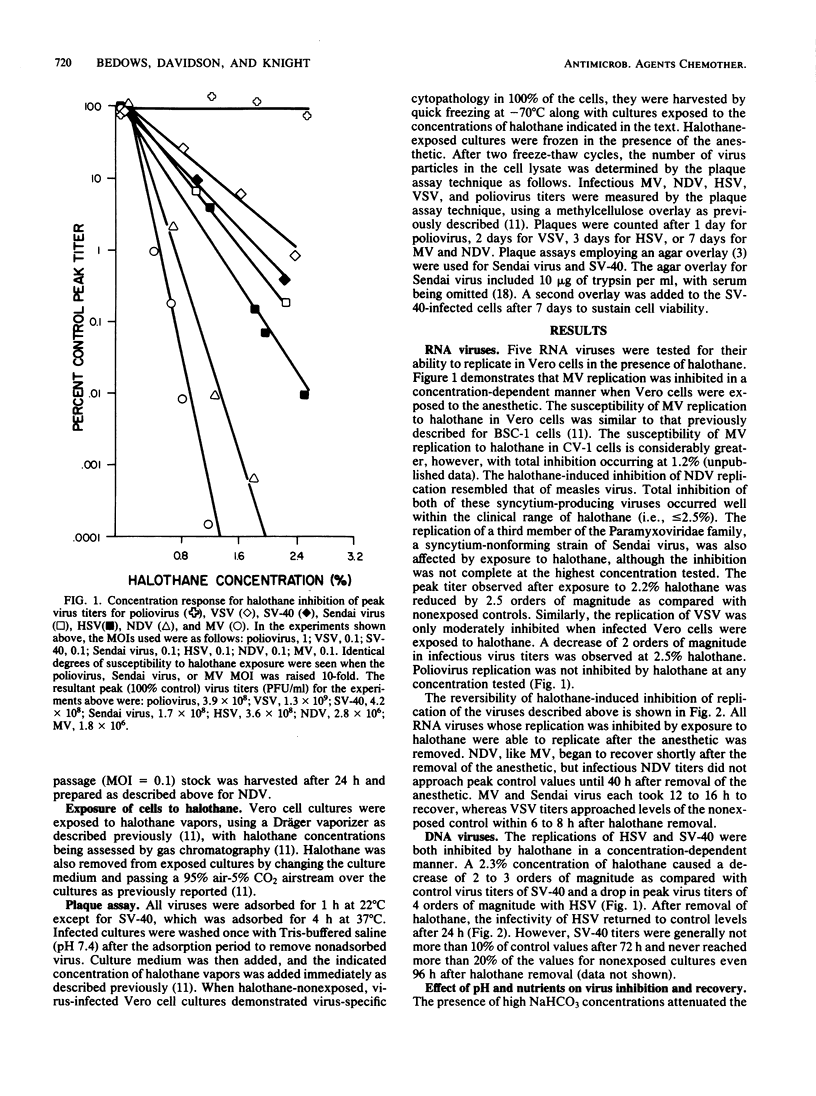
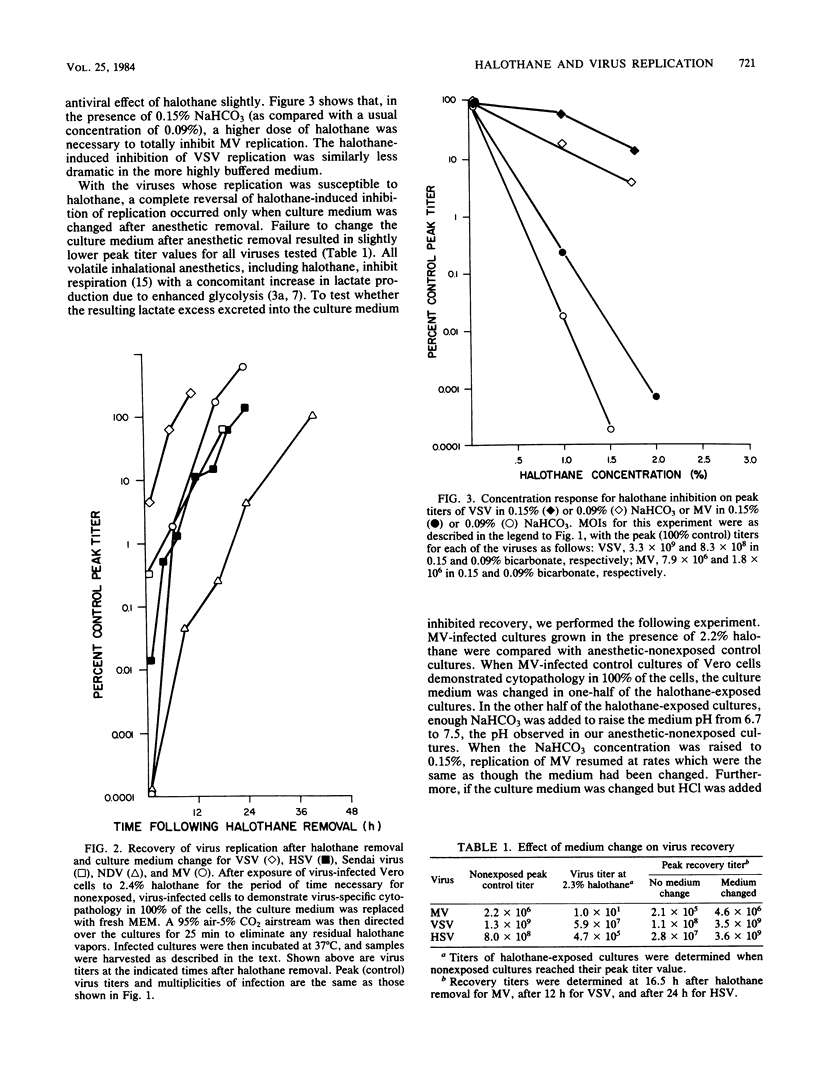



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedows E., Knight P. R. Pressure reversal of halothane's antiviral effect. Anesthesiology. 1983 Aug;59(2):109–112. doi: 10.1097/00000542-198308000-00007. [DOI] [PubMed] [Google Scholar]
- Bedows E., Payne F. E. Host cell factors involved in the production of slowly sedimenting nucleocapsids in measles virus-infected cells. J Virol. 1981 Jan;37(1):103–108. doi: 10.1128/jvi.37.1.103-108.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedows E., Rao K. M., Welsh M. J. Fate of microfilaments in vero cells infected with measles virus and herpes simplex virus type 1. Mol Cell Biol. 1983 Apr;3(4):712–719. doi: 10.1128/mcb.3.4.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabec M. J., Bedows E., Davidson B. A., Knight P. R. Effect of general anesthetics and pressure on aerobic metabolism of monkey kidney cells. Anesthesiology. 1984 Jul;61(1):43–47. [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Characterization of poliovirus-specific structures associated with cytoplasmic membranes. Virology. 1970 Sep;42(1):112–122. doi: 10.1016/0042-6822(70)90243-6. [DOI] [PubMed] [Google Scholar]
- Cohen P. J. Effect of hydrostatic pressure on halothane-induced depression of mitochondrial respiration. Life Sci. 1983 Apr 4;32(14):1647–1650. doi: 10.1016/0024-3205(83)90872-x. [DOI] [PubMed] [Google Scholar]
- Donovan C. A. The influence of diethyl ether inhalation on experimental canine distemper. Vet Med Small Anim Clin. 1968 Apr;63(4):345–347. [PubMed] [Google Scholar]
- Fink B. R., Kenny G. E. Metabolic effects of volatile anesthetics in cell culture. Anesthesiology. 1968 May-Jun;29(3):505–516. doi: 10.1097/00000542-196805000-00024. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Molecular mechanisms of general anaesthesia. Nature. 1982 Dec 9;300(5892):487–493. doi: 10.1038/300487a0. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Trudell J. R. Asymmetric antagonistic effects of an inhalation anesthetic and high pressure on the phase transition temperature of dipalmitoyl phosphatidic acid bilayers. Biochim Biophys Acta. 1980 Jun 20;599(1):336–340. doi: 10.1016/0005-2736(80)90080-2. [DOI] [PubMed] [Google Scholar]
- Knight P. R., Bedows E., Nahrwold M. L., Maassab H. F., Smitka C. W., Busch M. T. Alterations in influenza virus pulmonary pathology induced by diethyl ether, halothane, enflurane, and pentobarbital anesthesia in mice. Anesthesiology. 1983 Mar;58(3):209–215. doi: 10.1097/00000542-198303000-00001. [DOI] [PubMed] [Google Scholar]
- Knight P. R., Nahrwold M. L., Bedows E. Anesthetic action and virus replication: inhibition of measles virus replication in cells exposed to halothane. Antimicrob Agents Chemother. 1980 May;17(5):890–896. doi: 10.1128/aac.17.5.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight P. R., Nahrwold M. L., Bedows E. Inhibiting effects of enflurane and isoflurane anesthesia on measles virus replication: comparison with halothane. Antimicrob Agents Chemother. 1981 Sep;20(3):298–306. doi: 10.1128/aac.20.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M. J., Miller K. W., Paton W. D., Smith E. B. Pressure reversal of anaesthesia. Nature. 1971 Jun 11;231(5302):368–371. doi: 10.1038/231368a0. [DOI] [PubMed] [Google Scholar]
- Morgan E. M., Rapp F. Measles virus and its associated diseases. Bacteriol Rev. 1977 Sep;41(3):636–666. doi: 10.1128/br.41.3.636-666.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrwold M. L., Cohen P. J. Anesthetics and mitochondrial respiration. Clin Anesth. 1975;11(1):25–44. [PubMed] [Google Scholar]
- Schwartz J., Roizman B. Concerning the egress of herpes simplex virus from infected cells: electron and light microscope observations. Virology. 1969 May;38(1):42–49. doi: 10.1016/0042-6822(69)90126-3. [DOI] [PubMed] [Google Scholar]


