Abstract
Background and aims
The prognosis of metastatic colorectal cancer is still poor, raising the need for alternative therapeutic approaches, particularly by manipulating the antitumour immune response. Advanced tumour stages, however, are frequently accompanied by functional T cell defects which may be critical for a T cell based anticancer immunotherapy. The aim of this study was to address whether T cells from colorectal cancer patients with advanced tumour stages can be specifically antigen activated against their autologous tumour cells.
Methods
T cells were isolated from colorectal cancer patients and retrovirally transduced to express a recombinant immunoreceptor that has an extracellular binding domain for carcinoembryonic antigen (CEA) and an intracellular CD3ζ signalling domain with and without CD28 costimulation for T cell activation.
Results
Peripheral blood T cells from colorectal cancer patients were successfully engineered to express the anti‐CEA immunoreceptor on the cell surface. On coincubation with autologous CEA+ tumour cells, T cells with anti‐CEA immunoreceptor are specifically activated to secrete interferon γ (IFN‐γ) and to lyse autologous tumour cells whereas T cells without immunoreceptor are not. T cells equipped with combined CD3ζ‐CD28 signalling receptor are more efficiently activated to secrete IFN‐γ compared with T cells with CD3ζ signalling receptor. Induction of interleukin 2 secretion on targeting towards autologous tumour cells requires triggering of T cells by the CD3ζ‐CD28 costimulatory receptor.
Conclusions
T cells from advanced colorectal cancer patients can be tumour specifically activated with high efficiency by engraftment with a combined CD3ζ‐CD28 immunoreceptor to break tolerance against autologous tumour cells.
Keywords: immunotherapy, recombinant T cell receptor, CD28 costimulation, carcinoembryonic antigen
Despite continued advances in systemic and local treatment, colorectal cancer is still one of the common causes of cancer death in the Western world, with no significant improvement in overall five year survival.1 Many therapeutic options for advanced and metastatic colorectal cancer have evolved, most of which rely on adjuvant systemic chemotherapeutic regimens and surgery of hepatic metastases. As only 5–10% of patients have operable disease,2 alternative therapeutic approaches are currently being investigated, including gene therapy3 and immune therapeutic strategies.4 The latter include genetic manipulation of cells of the immune system, aiming to use the power and specificity of the cellular immune response for targeted elimination of cancer cells. In order to target T cell antigen specifically towards tumour cells, strategies were developed to equip T cells with a recombinant T cell receptor of defined specificity. In particular, the immunoreceptor strategy is based on grafting immunological effector cells with a recombinant receptor molecule (that is, immunoreceptor) that has a single chain antibody fragment (scFv) derived domain for antigen binding on its extracellular moiety and a CD3ζ signalling chain in its intracellular moiety for cellular activation.5 Binding of the immunoreceptor to antigen causes crosslinking of the receptor molecules and CD3ζ mediated downstream signalling, resulting in T cell activation with proliferation, induction of cytokine secretion, and cytolysis of the target cell. Work from our laboratories,6,8,9 along with others,10,11,12,13 has demonstrated that recombinant immunoreceptors can be engineered to activate cytotoxic T cell antigen specifically towards colorectal cancer cells by targeting carcinoembryonic antigen (CEA), CA72‐4 (TAG72), and CA19‐9, respectively, resulting in tumour cell lysis.
CEA (CD66e) is a 180 kDa glycoprotein and the name giving member of the CEA family which belongs to the large immunoglobulin superfamily. CEA is physiologically expressed on the luminal surface of epithelial cells of the gastrointestinal tract and respiratory organs and, moreover, on a variety of adenocarcinomas, such as of colon, rectum, pancreas, gastric, breast, and other organs. CEA is therefore well established as a tumour and serum marker for adenocarcinomas.14 In immunotherapy, different strategies have been developed to use CEA as a vaccine (for example, as recombinant CEA protein, CEA anti‐idiotypic antibodies, and dendritic cells pulsed with agonist CEA epitopes) to induce a CEA specific immune response.15 The presence of CEA on the tumour cell surface is utilised to target cytotoxic drugs or radioisotopes coupled to CEA specific antibodies to the tumour.16 Indirect technologies aim to use CEA as a target antigen for cytotoxic T cells (for example, by presenting CEA peptides bound to MHC class I alleles17 or by CEAxCD3 bispecific antibodies).18
However, a number of studies indicate that T cells from tumour bearing mice19 and from tumour patients20 are not functional with respect to T cell receptor (TCR) signalling due to altered expression of proteins of the CD3 complex. T cells infiltrating hepatic metastases of colorectal cancer have reduced CD3ζ chain expression and lack tumour specific activity in vitro.21 In view of the immunoreceptor concept, the question arises as to whether T cells from colorectal cancer patients can be specifically activated by receptor signalling on binding to tumour cells. Whereas previous studies demonstrated targeting of T cells from healthy donors towards tumour cells of established cell lines, we asked whether primary T cells from the peripheral blood of colorectal cancer patients in advanced stages can be specifically antigen activated by signalling through the recombinant immunoreceptor and whether these cells are activated on binding to autologous tumour cells derived from surgery specimens from the same patient. Hence our study addresses the question of whether engraftment with an immunoreceptor is capable of breaking antigen specific T cell tolerance against autologous colorectal cancer cells.
Materials and methods
Cell lines and reagents
293T cells are human embryonal kidney cells that express the SV40 large T antigen.7 LS174T is a CEA+ colorectal carcinoma line (ATCC CL‐188); Colo201 cells (ATCC CCL 224) were derived from an adenocarcinoma. OKT3 (ATCC CRL 8001) is a hybridoma cell line that produces the anti‐CD3 monoclonal antibody (mAb) OKT3. 293T cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal calf serum (FCS) and all other cell lines were cultured in RPMI 1640 medium, 10% (v/v) FCS (all Invitrogen Life Technologies, Paisley, UK). OKT3 mAb was affinity purified from hybridoma supernatants utilising goat antimouse IgG2a antibodies (Southern Biotechnology, Birmingham, Alabama, USA) that were immobilised on N‐hydroxy‐succinimide‐ester activated sepharose, as recommended by the manufacturer (Amersham Biosciences, Freiburg, Germany). Human IgG1 antibodies and the phycoerythrin (PE) conjugated anti‐CD3 mAb UCHT1 were purchased from Dako (Hamburg, Germany). The goat antihuman IgG antibody and its FITC and PE conjugated F(ab′)2 derivatives were purchased from Southern Biotechnology. The antihuman interferon γ (IFN‐γ) mAb NIB42 and the biotinylated antihuman IFN‐γ mAb 4S.B3 were purchased from BD Bioscience (San Diego, California, USA).
Isolation of tumour cells
Primary carcinoma cell cultures were established from colon carcinoma specimens by the single cell isolation procedure, as described previously.22 In brief, biopsy fragments were incubated in HBSS buffer containing 100 U/ml DNase I (Roche, Mannheim, Germany), 50 U/ml collagenase III (Biochrome, Berlin, Germany), 150 U/ml hyaluronidase (Sigma, Deisenhofen, Germany), and 0.08 U/ml insulin (Hoechst, Bad Soden, Germany) at 37° C for 15 minutes while shaking. Cells in the supernatant were collected by centrifugation for five minutes at 400 g, resuspended in 10 ml of erythrocyte lysation buffer (8.29 g/l NH4Cl, 1 g/l KHCO3, and 0.0371 g/l EDTA in Aqua dest; all from Sigma) and incubated at room temperature for 15 minutes. The erythrocyte depletion step was performed three times. Cells were washed and cultured at a density of 106–107 cells/ml in Leibovitz medium containing 10% (v/v) FCS, 1 mM L‐glutamine, 1×MEM vitamins, 2.5 mg/ml transferrin, 1 g/l sodium bicarbonate, 1 g/l glucose, 80 U/ml insulin, and 10 mg/ml gentamycin (all from Invitrogen), giving rise to a homogenous and adherently growing cell culture. Cultures contaminated with fibroblasts were discarded. Tumour cell cultures were checked for mycoplasma contamination and found to be free.
Immunocytochemical analysis
Cells grown in vitro were monitored for CEA expression by immunohistochemical analysis. Briefly, cells were spun onto microscope slides and fixed with ice cold acetone for 10 minutes. Non‐specific binding was blocked by 10% (v/v) FCS in phosphate buffered saline for 30 minutes. Slides were incubated for 60 minutes with either an anti‐CEA mAb (1C3; Abcam, Cambridge, Massachusetts, USA) or an isotype control mAb (BD Bioscience, San Jose, California, USA) (10 μg/ml). Bound antibodies were detected by a peroxidase conjugated Fab anti‐mouse Ab (1:50) (Roche Diagnostics) and visualised by 3‐Amino‐9‐ethylcarbazole (Sigma).
Recombinant immunoreceptors and FACS analysis
Generation of the expression cassettes for the CEA specific immunoreceptors BW431/26‐scFv‐Fc‐ζ and BW431/26‐scFv‐Fc‐CD28‐ζ (see figs 1A, 6A) was previously described. Retroviral transduction of T cells with the pBullet vector has been described in detail elsewhere.6,7 Recombinant receptor grafted T cells were identified by two colour immunofluorescence utilising a PE or FITC conjugated F(ab′)2 antihuman IgG1 antibody (1 μg/ml) and an FITC or PE conjugated anti‐CD3 mAb (UCHT‐1, 1:20). Immunofluorescence was analysed using a FACScan cytofluorometer equipped with the CellQuest research software (Becton Dickinson, Mountain View, California, USA). To identify T cells with recombinant receptor expression, we set markers with 99% of non‐transduced T cells beyond.
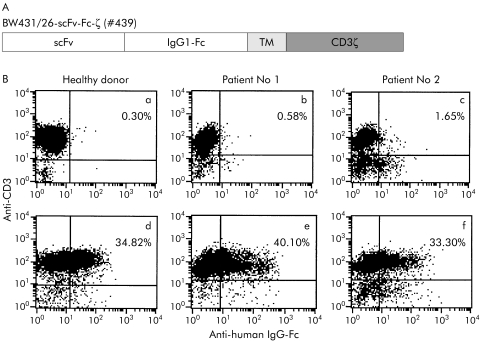
Figure 1 Expression of the recombinant carcinoembryonic antigen (CEA) specific immunoreceptor BW431/26‐scFv‐Fc‐ζ on the surface of T cells from colorectal cancer patients and from a healthy donor. (A) Schematic diagram of the expression cassette coding for the BW431/26‐scFv‐Fc‐ζ receptor (#439) used in this study. scFv, BW431/26 single chain antibody fragment; IgG1‐Fc, IgG1 CH2CH3; TM, transmembrane region; CD3ζ, CD3ζ intracellular chain with the signalling domains. (B) Lymphocytes were isolated from peripheral blood of colorectal patient Nos 1 and 2 and from a healthy donor (a–c) and transduced by retroviral gene transfer to express the BW431/26‐scFv‐Fc‐ζ receptor (d–f), as described in materials and methods. The receptor expressed on CD3+ T cells was recorded by two colour FACS analysis using the anti‐CD3 antibody OKT3 and the anti‐human IgG‐Fc antibody that detects the extracellular IgG1 CH2CH3 spacer domain of the receptor.
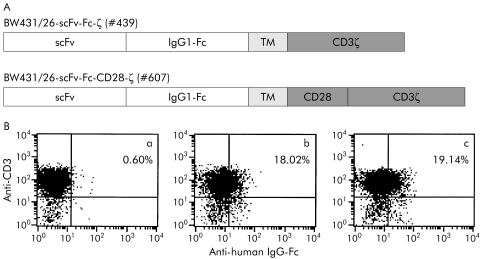
Figure 6 Lymphocytes from colorectal cancer patient No 3 were grafted with an anti‐carcinoembryonic antigen (CEA) receptor with combined CD3ζ and CD28 signalling domains. (A) Schematic diagram of the expression cassettes for the recombinant BW431/26‐scFv‐Fc‐ζ receptor (#439) with CD3ζ signalling domain and the BW431/26‐scFv‐Fc‐CD28‐ζ.receptor (#607) with the combined CD3ζ and CD28 signalling domains. scFv, BW431/26 single chain antibody fragment; IgG1‐Fc, IgG1 CH2CH3; TM, transmembrane region; CD3ζ, CD3ζ intracellular chain; CD28, CD28 intracellular chain. (B) Peripheral blood lymphocytes from colorectal patient No 3 (a) were retrovirally transduced to express the BW431/26‐scFv‐Fc‐ζ (b) and the BW431/26‐scFv‐Fc‐CD28‐ζ. receptor (c), respectively. FACS analysis using the anti‐CD3 antibody OKT3 and the anti‐human Ig‐Fc antibody that detects the extracellular IgG1 CH2CH3 spacer domain of the receptors demonstrates expression of the recombinant receptors on the surface of CD3+ T cells.
Receptor mediated activation of grafted T cells
T cells were grafted with recombinant anti‐CEA immunoreceptors and cocultivated in round bottom 96 well microtitre plates (1.25–10×104 grafted T cells/well) with CEA+ and CEA− tumour cells (5×104 cells/well), respectively. After 48 hours, culture supernatants were analysed by ELISA for IFN‐γ and interleukin (IL)‐2. Briefly, IFN‐γ was bound to the solid phase antihuman IFN‐γ mAb NIB42 (1 µg/ml) and detected by the biotinylated antihuman IFN‐γ mAb 4S.B3 (0.5 µg/ml). IL‐2 was bound by a solid phase anti‐IL‐2 antibody (1:250) and detected by a biotinylated anti‐human IL‐2 antibody (1:250) (both BD Bioscience). The reaction product was visualised by a peroxidase‐streptavidin conjugate (1:10,000) and ABTS (both Roche Diagnostics).
Specific cytotoxicity of receptor grafted T cells against target cells was monitored by a XTT based colorimetric assay.23 Briefly, XTT (2,3‐bis(2‐methoxy‐4‐nitro‐5sulphonyl)‐5[(phenyl‐amino)carbonyl]‐2H‐tetrazolium hydroxide) reagent (1 mg/ml) (Cell Proliferation Kit II; Roche Diagnostics) was added to the cells and incubated for 30–90 minutes at 37°C. Reduction of XTT to formazan by viable tumour cells was monitored colorimetrically at an adsorbance wavelength of 450 nm and a reference wavelength of 650 nm. Maximal reduction of XTT was determined as the mean of wells containing tumour cells only, and background as the mean of wells containing RPMI 1640 medium, 10% (v/v) FCS. The non‐specific formation of formazan due to the presence of effector cells was determined from triplicate wells containing effector cells in the same number as in the corresponding experimental wells. The number of viable tumour cells was calculated as follows:
Viability (%) = (OD(exp wells − corresponding number of effector cells) / OD(tumour cells without effectors − medium)) × 100
Results
We addressed the question of whether T cells from colorectal cancer patients can be specifically antigen activated towards their autologous tumour cells in vitro. In this study, we included three patients with advanced CEA+ colorectal cancer and surgical tumour resection. Patient details are listed in table 1.
Table 1 Patient characteristics.
| Patient No (ID) | Sex | Age (y) | Tumour | Tumour stage |
|---|---|---|---|---|
| 1 (HW) | F | 73 | Carcinoma of the sigmoid colon | Dukes' C |
| 2 (US) | F | 66 | Carcinoma of the sigmoid and rectum | Dukes' A |
| 3 (BS) | M | 50 | Rectum carcinoma | Dukes' D |
T cells were isolated from peripheral blood of colorectal cancer patients and from a healthy donor and retrovirally transduced in vitro to express the recombinant CEA specific immunoreceptor BW431/26‐scFv‐Fc‐ζ, as described in the materials and methods. Receptor expression on the surface of CD3+ T cells was monitored by FACS analysis using an anti‐IgG antibody directed against the extracellular IgG1 CH2CH3 domain of the receptor. As shown in fig 1B, approximately 30–40% of CD3+ T cells from the colorectal cancer patients expressed the recombinant immunoreceptor on the cell surface. For comparison, about 35% of T cells from a healthy donor expressed the immunoreceptor on the cell surface on retroviral transduction. At least in these examples, T cells from colorectal cancer patients were nearly equally efficiently transduced to express the immunoreceptor as T cells from the healthy individual.
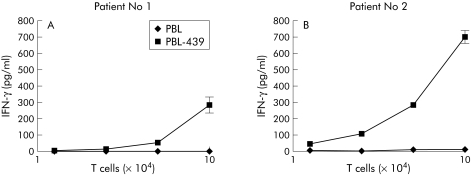
We determined whether immunoreceptor grafted T cells from colorectal cancer patients were specifically antigen activated on binding to CEA+ colorectal carcinoma cells. Thus lymphocytes from colorectal cancer patient Nos 1 and 2 were grafted with the CEA specific immunoreceptor BW431/26‐scFv‐Fc‐ζ and cocultured with cells of the CEA+ colorectal carcinoma line LS174T, respectively, and, as a control, with cells of the CEA− line Colo201. With an increasing ratio of engineered T cells to tumour cells, patient lymphocytes grafted with the BW431/26‐scFv‐Fc‐ζ receptor were induced to secrete increasing amounts of IFN‐γ whereas T cells without immunoreceptor were not activated (fig 2C, G). Induction of IFN‐γ secretion is due to interaction of CEA+ tumour cells with anti‐CEA receptor grafted T cells because neither coincubation of receptor grafted T cells with CEA negative Colo201 cells (fig 2D, H) nor of lymphocytes without the BW431/26‐scFv‐Fc‐ζ receptor (fig 2C, D, G, H) induced IFN‐γ secretion.
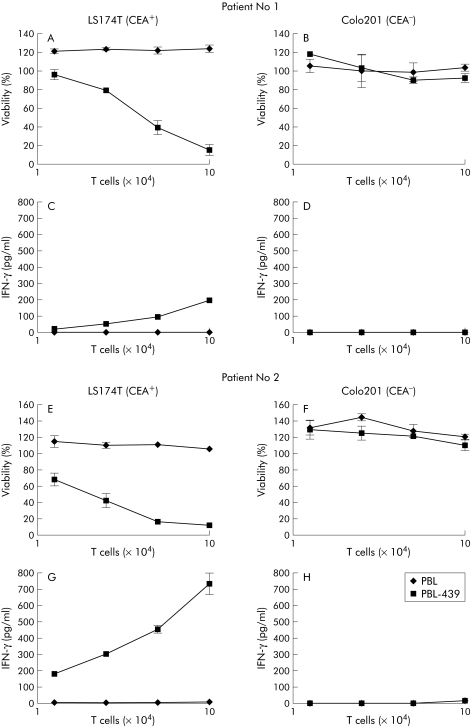
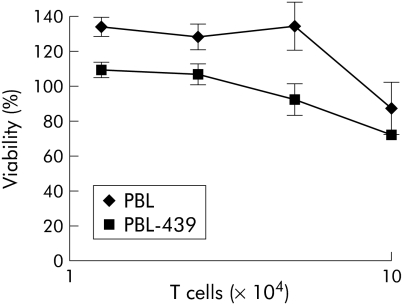
Figure 2 Antigen specific activation of patient T cells grafted with the BW431/26‐scFv‐Fc‐ζ immunoreceptor. Peripheral blood lymphocytes (PBL) from patient Nos 1 and 2 were grafted with the anti‐carcinoembryonic antigen (CEA) immunoreceptor BW431/26‐scFv‐Fc‐ζ (PBL‐439), as shown in fig 1B, and cocultivated for 48 hours (1.25–10×104 receptor grafted T cells/well) with CEA+ LS174T colorectal carcinoma and CEA− Colo201 tumour cells (each 2.5×104 cells/well). Patient's lymphocytes without BW431/26‐scFv‐Fc‐ζ receptor served as controls. Viability of target cells was determined colorimetrically by a tetrazolium salt based XTT assay (A, B, E, F) and the concentration of interferon γ (IFN‐γ) in culture supernatants was recorded by ELISA (C, D, G, H), as described in materials and methods.
To monitor the capacity of engineered T cells from colorectal cancer patients to lyse antigen specific established tumour cells, we recorded the viability of tumour cells in the presence of increasing numbers of engineered T cells using a XTT based colorimetric assay, as described in materials and methods. As summarised in fig 2, T cells grafted with the anti‐CEA immunoreceptor BW431/26‐scFv‐Fc‐ζ decreased the viability of CEA+ LS174T tumour cells whereas coincubation with T cells without immunoreceptor did not (fig 2A, E). CEA negative Colo201 tumour cells were not lysed by T cells either with or without anti‐CEA immunoreceptor (fig 2B, F). Taken together, this indicates that the cytolytic activity of receptor grafted T cells towards LS174T cells is mediated by the CEA specific immunoreceptor.
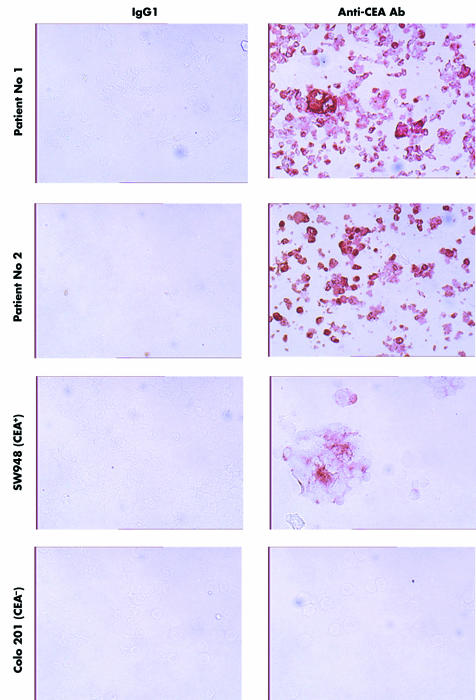
We addressed the question of whether autologous CEA+ tumour cells can be specifically targeted by T cells from colorectal cancer patients. We therefore isolated tumour cells from colorectal carcinoma specimens obtained by surgery from the same patient Nos 1 and 2. During short term cultivation in vitro, the majority of colorectal carcinoma cells from both patients continued to express CEA at high density on the cell surface (fig 3). For comparison, established SW948 colorectal carcinoma cells expressed CEA at lower levels and Colo201 cells did not express detectable amounts of CEA.
Figure 3 Primary tumour cells isolated from colorectal cancer biopsies expressed carcinoembryonic antigen (CEA) in vitro. Tumour cells were isolated from tumour specimens obtained during surgery and cultured for short time periods in vitro. Expression of CEA was monitored by means of immunohistochemical procedures using the anti‐CEA antibody 1C3, as described in material and methods. Note the natural heterogeneity of CEA expression within the tumour cell population. Monitoring of CEA on established CEA+ LS174T colorectal cancer cells and CEA− Colo201 cells is shown for comparison.
To test whether immunoreceptor grafted T cells from colorectal cancer patients are specifically CEA activated by their autologous tumour cells, we coincubated increasing numbers of BW431/26‐scFv‐Fc‐ζ receptor grafted lymphocytes from patient Nos 1 and patient 2, respectively, with the autologous CEA+ tumour cells. As shown in fig 4, T cells with BW431/26‐scFv‐Fc‐ζ immunoreceptor were dose dependently induced to secrete increasing amounts of IFN‐γ on coincubation with the autologous tumour cells whereas T cells from the same patients without receptor did not. Obviously, T cells from colorectal cancer patients and grafted with anti‐CEA immunoreceptor are specifically CEA activated on binding to their autologous tumour cells. To test whether T cell activation includes induction of cytolysis, we recorded the viability of patient tumour cells on incubation with autologous T cells equipped with or without BW431/26‐scFv‐Fc‐ζ receptor. As illustrated in fig 5, increasing numbers of BW431/26‐scFv‐Fc‐ζ receptor grafted T cells lysed the autologous CEA+ tumour cells in a dose dependent fashion whereas T cells without immunoreceptor did not. Taken together, the data demonstrate that patient T cells with the BW431/26‐scFv‐Fc‐ζ immunoreceptor are activated to IFN‐γ secretion and cytolysis on binding to autologous CEA+ tumour cells. This moreover indicates that engraftment with the CEA specific immunoreceptor breaks T cell tolerance of colorectal cancer patients towards their autologous CEA+ tumour cells.
Figure 4 Engineered patient's T cells with anti‐carcinoembryonic antigen (CEA) receptor were specifically activated by autologous tumour cells. Lymphocytes from peripheral blood (PBL) of colorectal cancer patient Nos 1 and 2 were grafted with the anti‐CEA immunoreceptor BW431/26‐scFv‐Fc‐ζ (PBL‐439) (see fig 1B), and 1.25–10×104 grafted cells per well were coincubated with autologous CEA+ primary tumour cells (5×104 cells/well) for 48 hours. PBL without BW431/26‐scFv‐Fc‐ζ receptor served as controls. Interferon γ (IFN‐γ) in the culture supernatant was determined by ELISA.
Figure 5 Patient's lymphocytes grafted with anti‐carcinoembryonic antigen (CEA) receptor lysed autologous CEA+ tumour cells. Lymphocytes from peripheral blood (PBL) of patient No 1 were grafted with the BW431/26‐scFv‐Fc‐ζ receptor (PBL‐439) and coincubated (1.25–10×104 cells/well) with autologous CEA+ tumour cells (5×104 cells/well) for 48 hours. Coincubation with PBL without BW431/26‐scFv‐Fc‐ζ receptor served as control. Viability of tumour cells was determined colorimetrically by a tetrazolium salt based XTT assay, as described in materials and methods.
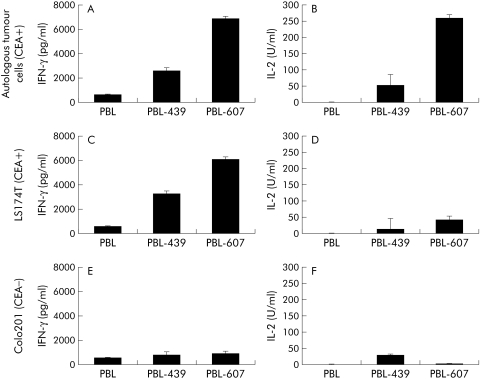
We then determined whether activation of T cells from colorectal cancer patients could be increased by CD28 costimulation simultaneously with CD3ζ signalling by the immunoreceptor. We therefore grafted, by retroviral gene transfer, T cells from patient No 3 with the BW431/26‐scFv‐Fc‐ζ receptor and the BW431/26‐scFv‐Fc‐CD28‐ζ receptor, respectively, that harbours the intracellular CD28 signalling domain in addition to the CD3ζ domain (fig 6A, B). Coincubation of receptor grafted T cells with CEA+ LS174T tumour cells specifically induced IFN‐γ and IL‐2 secretion (fig 7). Cyokine secretion however was significantly increased on T cell stimulation via the BW431/26‐scFv‐Fc‐CD28‐ζ receptor, with combined CD28 and CD3ζ signalling domains compared with stimulation via the CD3ζ signalling receptor without the CD28 costimulatory domain (see fig 7C, D). T cell activation was CEA specific because coincubation with CEA negative Colo201 tumour cells did not substantially induce IFN‐γ or IL‐2 secretion (fig 7E, F). To address whether receptor mediated CD28 costimulation also increases T cell activation towards autologous tumour cells, we coincubated primary autologous CEA+ tumour cells together with receptor grafted T cells from the same patient. As shown in fig 7A and fig 7B, T cells grafted with the BW431/26‐scFv‐Fc‐CD28‐ζ receptor secreted substantially more IFN‐γ and IL‐2 than T cells grafted with the BW431/26‐scFv‐Fc‐ζ receptor with the CD3ζ signalling domain only. Moreover, receptor triggered CD28 costimulation simultaneously with CD3ζ signalling is required to induce high IL‐2 secretion of grafted T cells (fig 7B). This set of analyses demonstrates that receptor mediated activation of T cells from colorectal cancer patients towards autologous tumour cells can be significantly increased by signalling through the combined C3ζ‐CD28 costimulatory receptor.
Figure 7 Enhanced activation of T cells from a colorectal cancer patient against autologous tumour cells by the combined CD3ζ‐CD28 costimulatory receptor. Lymphocytes from peripheral blood (PBL) of colorectal cancer patient No 3 were grafted by retroviral gene transfer with the carcinoembryonic antigen (CEA) specific receptors BW431/26‐scFv‐Fc‐ζ.(#439) and BW431/26‐scFv‐Fc‐CD28‐ζ (#607), respectively (see fig 6B). Receptor grafted lymphocytes (PBL‐439; PBL‐607) as well as non‐transduced lymphocytes (PBL) from the same patient (1×105 cells/well) were coincubated for 48 hours with autologous primary CEA+ colon cells (A, B), CEA+ LS174Τ colorectal tumour cells (C, D), and CEA− Colo201 (E, F) (5×104 cells/well each), respectively. Interferon γ (IFN‐γ) (A, C, E) and interleukin 2 (IL‐2) (B, D, F) were monitored in the culture supernatants by ELISA.
Discussion
Previous studies from our laboratory and others demonstrated that T cells can be grafted with specificity for CEA by expression of a recombinant immunoreceptor on the cell surface.6,10,11,12,24 The recombinant BW431/26‐scFv‐Fc‐ζ receptor used in this study harbours the BW431/26 scFv domain in the extracellular moiety for CEA binding and the intracellular CD3ζ signalling domain for T cell activation. The vast majority of studies to date have used T cells from healthy donors to demonstrate the feasibility of receptor mediated redirecting of T cells. In contrast with T cells from healthy donors, T lymphocytes from cancer patients were reported to be defective in the T cell activation machinery.20,21,25 For clinical application of the immunoreceptor concept however, it is crucial as to whether patient T cells can be effectively activated resulting in efficient redirecting towards autologous tumour cells. The data presented here however demonstrate that the conditions used to transduce by retroviral gene transfer T cells from patients with advanced colorectal cancer, at least in these examples, are nearly equally effective as for T cells from healthy donors (see fig 1). On binding to antigen, the BW431/26‐scFv‐Fc‐ζ receptor drives activation of patient T cells similarly effective as that of healthy donor T cells with respect to IFN‐γ secretion and lysis of CEA+ LS174T tumour cells (see fig 2). Receptor driven T cell activation is antigen specific because CEA negative Colo201 cells are not lysed in the presence of receptor grafted T cells and IFN‐γ secretion by grafted T cells is not induced. This demonstrates very well that T cells from colorectal cancer patients can be activated against CEA+ tumour cells by grafting with the appropriate immunoreceptor.
Moreover, our study aims were to elucidate whether patient T cells can be redirected towards autologous colon carcinoma cells. The tumour cells we isolated from surgical specimens and cultured in vitro for short time periods continued to express CEA on their cell surface, as shown by immunohistochemical techniques. T cells isolated from peripheral blood of these patients and grafted with the BW431/26‐scFv‐Fc‐ζ receptor were efficiently activated against autologous tumour cells in vitro, indicated by an increase in IFN‐γ secretion and cytolysis of tumour cells (see fig 4). Tumour cell lysis by receptor grafted T cells, in contrast, was only slightly greater than mediated by non‐modified lymphocytes (fig 5) which may be due to the heterogeneous expression of CEA on the tumour cell surface and to tumour cells that lost CEA expression. Natural heterogeneity, and particularly loss of CEA expression however, is a significant limitation of the immunoreceptor strategy that is based on antigen directed target recognition. This limitation may be overcome by targeting more than one antigen on the tumour cell surface or by recruiting bystander T or NK cells via secreted cytokines.
T cell activation was more efficient when T cells were grafted with the BW431/26‐scFv‐Fc‐CD28‐ζ receptor with the costimulatory CD28 domain in addition to the CD3ζ domain compared with the BW431/26‐scFv‐Fc‐ζ receptor without the CD28 domain (fig 7). Transduction efficiencies and densities of the receptors on the T cell surface were however nearly similar. IL‐2 secretion by patient T cells, moreover, was significantly induced by the BW431/26‐scFv‐Fc‐CD28‐ζ costimulatory receptor in contrast with the BW431/26‐scFv‐Fc‐ζ receptor, as previously shown in allogeneic systems.26 Enhanced IL‐2 secretion triggered by the costimulatory receptor will furthermore result in improved recruiting of cytolytic bystander cells to the tumour site. Taken together, the study here illustrates well that tolerance of peripheral blood T cells from colorectal cancer patients towards autologous tumour cells can be antigen specifically overcome by grafting with a CEA specific immunoreceptor that drives T cell activation on binding to CEA.
Soluble antigen in the serum of cancer patients, particularly in advanced stages, may prevent receptor mediated activation of grafted T cells by blocking the recombinant immunoreceptor. In the case of the BW431/26‐scFv‐Fc‐ζ receptor, we previously demonstrated27 that the presence of soluble CEA in concentrations up to 20 μg/ml does not block receptor mediated T cell activation and does not inhibit induction of cytolytic activities. This suggests that high serum CEA levels will not interfere with the activity of the anti‐CEA immunoreceptor in colorectal cancer patients. Our data on BW431/26‐scFv‐Fc‐ζ receptor driven T cell activation are in line with the recombinant anti‐CEA receptor MFE23.CD3ζTDGA reported by Gilham and colleagues.24
In vitro studies performed here, although including primary tumour cell isolates and autologous T cells, do certainly not reflect in toto the natural tumour microenvironment during a T cell attack. For example, tumour cells frequently upregulate expression of death receptor ligands and produce a panel of cytokines, such as transforming growth factor β or IL‐10, which inhibit T cell activation. Whereas these mechanisms may be partially active in the in vitro system, tumour secreted cytokines will be significantly diluted during culture of disaggregated tumour cells and will not accumulate to those concentrations present locally in the near vicinity of the tumour cells. Most importantly, tumour cells in these assays are not embedded into their network of stromal cells protecting the tumour cells from an immunological attack and providing the architectural superstructure that facilitates tumour growth. For an effective antitumour response, it is anticipated that T cells need to infiltrate the stromal network and to get in close contact with the tumour cells to allow tumour cell lysis, to proliferate, and to secrete cytokines that in turn may recruit and/or activate additional effector cells.
Our study is in line with previous studies from our group and others using T cells from cancer patients and autologous tumour cells.17,28,29 We reported29 that T cells from a patient with CD30+ cutaneous lymphoma can be effectively redirected in vitro towards the autologous tumour cells freshly isolated from a tumour biopsy resulting in highly efficient tumour cell lysis. Sheen and colleagues28 demonstrated the efficient transduction of T cells from colorectal cancer patients to express an anti‐CEA immunoreceptor and successful activation of T cells against autologous tumour cells. These reports together with the data presented here clearly demonstrate that T cells from tumour patients can be genetically manipulated by grafting with a recombinant immunoreceptor to break tolerance against autologous tumour cells. The recombinant BW431/26‐scFv‐Fc‐CD28‐ζ receptor with combined CD28 and CD3ζ signalling domains used in this study substantially enhances the cellular immune response against autologous tumour cells, thereby counteracting induction of tolerance against autologous tumour cells. This represents an important step towards the development of the immunoreceptor strategy for use in the immunotherapy of malignant diseases by specific manipulation of effector cells.
Acknowledgements
Work in the Cologne Laboratory is supported by grants from the Deutsche Forschungsgemeinschaft, the Deutsche Krebshilfe‐Mildred Scheel‐Stiftung, Bonn, and the Wilhelm Sander‐Stiftung, Munich.
Abbreviations
CEA - carcinoembryonic antigen
scFv - single chain antibody fragment
mAb - monoclonal antibody
TCR - T cell receptor
XTT - 2,3‐bis(2‐methoxy‐4‐nitro‐5sulphonyl)‐5[(phenyl‐amino)carbonyl]‐2H‐tetrazolium hydroxide
FCS - fetal calf serum
PE - phycoerythrin
IFN‐γ - interferon γ
IL - interleukin
Footnotes
Conflict of interest: None declared.
References
- 1.Midgley R, Kerr D. Colorectal cancer. Lancet 1999353391–399. [DOI] [PubMed] [Google Scholar]
- 2.Primrose J N. Treatment of colorectal metastases:surgery, cryotherapy, or radiofrequency ablation. Gut 2002501–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen M H, Chung‐Faye G A, Searle P F.et al Gene therapy for colorectal cancer: therapeutic potential. Bio Drugs 200115357–367. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell M S. Cancer vaccines, a critical review—part I. Curr Opin Investig Drugs 20023140–149. [PubMed] [Google Scholar]
- 5.Thistlethwaite F, Mansoor W, Gilham D E.et al Engineering T‐cells with antibody‐based chimeric receptors for effective cancer therapy. Curr Opin Mol Ther 2005748–55. [PubMed] [Google Scholar]
- 6.Hombach A, Schneider C, Sent D.et al An entirely humanized CD3 ζ‐chain signaling receptor that directs peripheral blood T cells to specific lysis of carcinoembryonic antigen‐positive tumor cells. Int J Cancer 200088115–120. [PubMed] [Google Scholar]
- 7.Weijtens M E, Willemsen R A, Hart E H.et al A retroviral vector system “STITCH” in combination with an optimized single chain antibody chimeric receptor gene structure allows efficient gene transduction and expression in human T lymphocytes. Gene Ther 199851195–1203. [DOI] [PubMed] [Google Scholar]
- 8.Hombach A, Heuser C, Sircar R.et al T cell targeting of TAG72+ tumor cells by a chimeric receptor with antibody‐like specificity for a carbohydrate epitope. Gastroenterology 19971131163–1170. [DOI] [PubMed] [Google Scholar]
- 9.Heuser C, Hombach A, Lösch C.et al T‐cell activation by recombinant immunoreceptors: impact of the intracellular signalling domain on the stability of receptor expression and antigen‐specific activation of grafted T cells. Gene Ther 2003101408–1419. [DOI] [PubMed] [Google Scholar]
- 10.Nolan K F, Yun C O, Akamatsu Y.et al Bypassing immunization: optimized design of “designer T cells” against carcinoembryonic antigen (CEA)‐expressing tumors, and lack of suppression by soluble CEA. Clin Cancer Res 199953928–3941. [PubMed] [Google Scholar]
- 11.Beecham E, Ortiz‐Pukola S, Junghans R. Dynamics of tumor cell killing by human T lymphocytes armed with an anti‐cancrinoembryonic antigen chimeric immunoglobulin T‐cell receptor. J Immunother 200023332–343. [DOI] [PubMed] [Google Scholar]
- 12.Daly T, Royal R E, Kershaw M H.et al Recognition of human colon cancer by T cells transduced with a chimeric receptor gene. Cancer Gene Ther 20007284–291. [DOI] [PubMed] [Google Scholar]
- 13.Sheen A J, Sherlock D J, Irlam J.et al T lymphocytes isolated from patients with advanced colorectal cancer are suitable for gene immunotherapy approaches. Br J Cancer 2003881119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballesta A M, Molina R, Filella X.et al Carcinoembryonic antigen in staging and follow‐up of patients with solid tumors. Tumor Biol 19951632–41. [DOI] [PubMed] [Google Scholar]
- 15.Sarobe P, Huarte E, Lasarte J J.et al Carcinoembryonic antigen as a target to induce anti‐tumor immune responses. Curr Cancer Drug Targets 20044443–454. [DOI] [PubMed] [Google Scholar]
- 16.Goldenberg D M. Carcinoembryonic antigen as a target cancer antigen for radiolebeled antibodies: prospects for cancer imaging and therapy. Tumor Biol 19951662–73. [DOI] [PubMed] [Google Scholar]
- 17.Bremers A J A, Vanderburg S H, Kuppen P J K.et al The use of Epstein‐Barr virus‐transformed B lymphocyte cell lines in a peptide‐reconstitution assay—identification of CEA‐related HLA‐A*0301‐restricted potential cytotoxic T‐lymphocyte epitopes. J Immunother 19951877–85. [DOI] [PubMed] [Google Scholar]
- 18.Kuwahara M, Arakawa F, Senba T.et al A mouse/human‐chimeric bispecific antibody reactive with human carcinoembryonic antigen‐expressing cells and human T lymphocytes. Anticancer Res 1996162661–2667. [PubMed] [Google Scholar]
- 19.Mizoguchi H, O'Shea J J, Longo D L.et al Alterations in signal tansduction molecules in T lymphocytes from tumor‐bearing mice. Science 19922581795–1798. [DOI] [PubMed] [Google Scholar]
- 20.Bukowski R M, Rayman T, Uzzo R.et al Signal transduction abnormalitires in T lymphocytes from patients with advanced renal carcinoma: clinical relevance and effects of cytokine therapy. Clin Cancer Res 199842337–2347. [PubMed] [Google Scholar]
- 21.Yoong K F, Adams D H. Interleukin‐2 restores CD3‐zeta chain expression but fails to generate tumour‐specific lytic activity in tumour‐infiltrating lymphocytes derived from human colorectal haptic metastases. Br J Cancer 1998771072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kemmner W, Schlag P, Brossmer R. A rapid and simple procedure for dissociation of tumor tissue from the human colon. J Cancer Res Clin Oncol 1987113400–401. [DOI] [PubMed] [Google Scholar]
- 23.Jost L M, Kirkwood J M, Whiteside T L. Improved short‐ and long term XTT‐based colorimetric cellular cytotoxicity assay for melanoma and other tumor cells. J Immunol Methods 1992147153–165. [DOI] [PubMed] [Google Scholar]
- 24.Gilham D E, O'Neil A, Hughes C.et al Primary polyclonal human T lymphocytes targeted to carcino‐embryonic antigen and neural cell adhesion molecule tumor antigens by CDz‐based chimeric immune receptors. J Immunother 200225139–151. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg S, Yannelli J, Topalian S.et al Treatment of patients with metatatic melanoma with autologous tumour‐infiltrating lymphocytes and interleukin‐2. J Nat Cancer Inst 1994861159–1166. [DOI] [PubMed] [Google Scholar]
- 26.Hombach A, Wieczarkowiecz A, Marquardt T.et al Tumor‐specific T cell activation by recombinant immunoreceptors: CD3ζ signaling and CD28 co‐stimulation are simultaneously required for efficient IL‐2 secretion and can be integrated into one combined CD28/CD3ζ signaling receptor molecule. J Immunol 20011676123–6131. [DOI] [PubMed] [Google Scholar]
- 27.Hombach A, Koch D, Sircar R.et al A chimeric receptor that selectively targets membrane‐bound carcinoembryonic antigen (mCEA) in the presence of soluble CEA. Gene Ther 19996300–304. [DOI] [PubMed] [Google Scholar]
- 28.Sheen A J, Irlam J, Kirillova N.et al Gene therapy of patient‐derived T lymphocytes to target and eradicate colorectal hepatic metastases. Dis Colon Rectum 200346793–804. [DOI] [PubMed] [Google Scholar]
- 29.Hombach A, Muche J M, Gerken M.et al T cells engrated with a recombinant anti‐CD30 receptor target autologous CD30+ cutaneous lymphoma cells. Gene Ther 20018891–895. [DOI] [PubMed] [Google Scholar]