Abstract
Backgrounds and aims
Controversy still exists as to whether gastrointestinal colonisation by Candida albicans contributes to aggravation of atopic dermatitis. We hypothesised that Candida colonisation promotes food allergy, which is known to contribute to a pathogenic response in atopic dermatitis. We tested this using a recently established murine Candida colonisation model.
Methods
Candida colonisation in the gastrointestinal tract was established by intragastric inoculation with C albicans in mice fed a synthetic diet. To investigate sensitisation against food antigen, mice were intragastrically administered with ovalbumin every other day for nine weeks, and antiovalbumin antibody titres were measured weekly. To examine gastrointestinal permeation of food antigen, plasma concentrations of ovalbumin were measured following intragastric administration of ovalbumin.
Results
Ovalbumin specific IgG and IgE titres were higher in BALB/c mice with Candida colonisation than in normal mice. Gastrointestinal permeation of ovalbumin was enhanced by colonisation in BALB/c mice. Histological examination showed that colonisation promoted infiltration and degranulation of mast cells. Candida colonisation did not enhance ovalbumin permeation in mast cell deficient W/Wv mice but did in congenic littermate control +/+ mice. Reconstitution of mast cells in W/Wv mice by transplantation of bone marrow derived mast cells restored the ability to increase ovalbumin permeation in response to Candida colonisation.
Conclusions
These results suggest that gastrointestinal Candida colonisation promotes sensitisation against food antigens, at least partly due to mast cell mediated hyperpermeability in the gastrointestinal mucosa of mice.
Keywords: Candida albicans , food allergy, atopic dermatitis, mast cells
Candida albicans is part of the indigenous microbial flora of the human gastrointestinal tract. In healthy individuals, populations of this fungus pose no threat to the host. However, in hosts receiving antibiotic treatment,1 immunocompromised states,2,3,4 and occasionally in apparently healthy persons,5 elevated populations can pose a significant risk.5 In addition, it has been hypothesised that excessive colonisation by C albicans in the gastrointestinal tract may constitute aggravating factors in atopic dermatitis (AD), but this remains controversial.4,6,7 AD is a chronic, relapsing, highly pruritic inflammatory skin disease with multifactorial causes, such as susceptibility genes, conditions within the host environment, and immunological factors.8 To date, laboratory and clinical investigations have demonstrated that IgE mediated food allergy plays a pathogenic role in a subset of patients with AD.9,10,11 Some reports have shown increased gastrointestinal permeability in AD patients.12,13,14 Hyperpermeability of the gastrointestinal mucosal barrier results in enhanced transport of intact and degraded antigens across the gastrointestinal mucosal barrier, which could favour food protein sensitisation and food allergy in susceptible individuals.15 We therefore hypothesised that gastrointestinal colonisation by C albicans may be involved in aggravation of AD by affecting the mucosal barrier in a manner that results in increased permeation of food allergens and subsequent manifestation of a food allergy.
Most models of gastrointestinal colonisation by C albicans have used oral inoculation of C albicans in adult mice treated with antibiotics and immunosuppressive agents,16,17,18,19,20,21 or in infant mice.22,23 These treatment regimens have been necessary because competitive indigenous bacterial flora and the immune system prevent colonisation by C albicans.17,19,24 However, given that C albicans is indigenous to the gastrointestinal tract of healthy humans, such methods of administration, particularly the immunosuppressive route, should be avoided so that an animal model that is typical of gastrointestinal colonisation by C albicans can be developed and the relationship between gastrointestinal Candida colonisation and allergic responses can be studied further. We recently reported a model of sustained gastrointestinal Candida colonisation by a single intragastric inoculation of C albicans in healthy adult mice without administration of antibiotics or immunosuppresants.25 This was achieved by feeding mice a synthetic diet that resulted in reduced numbers of lactobacilli in the stomach. While animals show a good healthy appearance in this model, high faecal recovery of C albicans is still observed at least 18 months after the single inoculation (unpublished data), suggesting asymptomatic Candida colonisation in the gastrointestinal tract. Therefore, using this model it was possible to investigate the role of gastrointestinal colonisation by C albicans in the sensitisation against food allergens.
To test our hypothesis, we initially determined whether sensitisation against food antigens was promoted in mice with gastrointestinal tracts that had been chronically colonised by C albicans. We then assessed the effect of gastrointestinal Candida colonisation on permeation of food antigens. Finally, because the barrier and transport properties of the gastrointestinal epithelium are actively regulated by mast cells which are activated in response to various pathogen associated stimuli,26,27 we investigated whether mast cells were involved in the permeation of food antigens in mice with gastrointestinal Candida colonisation.
Materials and methods
Animals
Specific pathogen free female five week old mice were used in all experiments. BALB/c mice were purchased from Charles River Japan (Yokohama, Japan). Mast cell deficient mice (WBB6F1‐W/Wv) and congenic littermate control mice (WBB6F1‐+/+) were purchased from Japan SLC (Hamamatsu, Japan). All mice were housed in a temperature controlled (23±2°C) room with a dark period from 20:00 to 08:00 and allowed free access to water and a purified diet prepared according to AIN‐93G.28 The study was approved by the Hokkaido University Animal Use Committee, and animals were maintained in accordance with the guidelines for the care and use of laboratory animals of Hokkaido University.
Inoculation and enumeration of C albicans
C albicans (JCM 1542) was obtained from Japan Collection of Microorganisms of the Institute of Physical and Chemical Research (Saitama, Japan) and maintained as previously described.25 For inoculation, all mice were acclimatised to the purified diet for two weeks before being deprived of the diet for 16 hours. Mice were then inoculated intragastrically with 0.2 ml of saline containing 1×108 cells of C albicans. Control mice were intragastrically administered 0.2 ml of the vehicle. Faecal specimens and tissue homogenates were quantitatively cultured using a standard pour plate technique, as previously described.25
Oral immunisation experiment
At three weeks after inoculation, oral immunisation with ovalbumin (OVA) was started in BALB/c mice. Phosphate buffered saline (PBS 0.2 ml) containing 0.1 mg of OVA (grade V; Sigma, Missouri, USA) was intragastrically administered every other day for nine weeks. Blood samples were obtained from the tail vein at weekly intervals and subjected to ELISA for measurement of OVA specific antibody titres, as described below. In addition, every week, faecal specimens were analysed for number of C albicans organisms, as described above. On the last day of the experiment, mice were anaesthetised with diethyl ether and sacrificed by exsanguination from the carotid artery. Following laparotomy, the stomach, jejunum, ileum, and colon were excised, homogenised in PBS, and the number of C albicans organisms were counted, as described above.
Gastrointestinal permeation experiment
Gastrointestinal permeability was determined in vivo by measuring the appearance in blood of horseradish peroxidase (HRP) and OVA, administered by gavage according to Wang and colleagues29 and Saitoh and colleagues,30 respectively, with some modifications.
At four to five weeks after inoculation, mice were deprived of the diet for 12 hours, after which 0.6 ml of PBS containing 0.6 mg of HRP (Sigma) or 0.2 ml of PBS containing 2 mg of OVA were intragastrically administered. Blood samples were then collected from the tail vein at 0, 30, 60, and 120 minutes after administration, and plasma HRP and OVA concentrations were measured by ELISA, as described below. Thereafter, mice were anaesthetised with diethyl ether and sacrificed by exsanguination from the carotid artery. Following laparotomy, the stomach was excised, opened along the greater curvature, washed with ice cold saline, and embedded in OCT compound (Sakura Finetechnical, Tokyo, Japan) for histological examination.
ELISA
For determination of OVA specific IgG titre in the sera of mice, 96 well microtitre plates (Corning, New York, USA) were coated overnight at 4°C with 200 μg/ml OVA in 50 mmol/l carbonate buffer, pH 9.6. Plates were blocked with PBS containing 1% bovine serum albumin (BSA, fraction V; Serologicals Proteins, Illinois, USA) (PBS‐B) at 37°C for one hour. Test sera serially diluted with PBS containing 0.2% BSA and 0.02% Tween 20 (PBS‐BT) were then added and incubated at 37°C for two hours. After incubation, HRP conjugated goat antimouse IgG (Zymed, California, USA), diluted 1:2000 in PBS‐BT, was added and incubated at 37°C for one hour. Wells were washed five times with PBS containing 0.02% Tween 20 (PBS‐T) between each step. Plates were developed at room temperature after addition of o‐phenylendiamine (0.4 mg/ml) and hydrogen peroxide (0.012%) in 7 mmol/l citrate buffer, pH 5.0. Finally, 1 mol/l H2SO4 was added, and absorbance was measured at 490 nm with a microplate reader (model 550; Bio‐Rad, California, USA). Preimmunised serum was used as a negative control. The average extinction in negative control wells, to which three times the standard deviation was added, provided the reference for determination of the titre in the test sera. Antibody titres were expressed as the reciprocal of the last dilution yielding an extinction value higher than the reference value.
For determination of the OVA specific IgE titre, 96 well microtitre plates were coated overnight at 4°C with 10 μg/ml antimouse IgE monoclonal antibody (LO‐ME‐2; Zymed) in 50 mmol/l carbonate buffer, pH 9.6. Plates were blocked with PBS‐B at 37°C for one hour. Test sera serially diluted with PBS‐BT were then added and incubated overnight at 4°C. After incubation, 20 ng/ml OVA‐DIG conjugate in PBS‐BT was added and incubated at 37°C for one hour. Coupling of DIG to OVA was performed using a DIG protein labelling kit (Roche Diagnostics, Tokyo, Japan) according to the manufacturer's instructions. HRP conjugated sheep anti‐DIG Fab fragments (Roche Diagnostics), diluted 1:4000 in PBS‐BT, was then added and incubated at 37°C for one hour. Wells were washed five times with PBS‐T between each step. Plates were developed at room temperature after addition of 3,3′,5,5′‐tetramethylbenzidine (0.123 mg/ml; Sigma) and hydrogen peroxide (0.012%) in 2 mol/l citrate buffer, pH 5.0. Finally, 1 mol/l H2SO4 was added, and absorbance was measured at 450 nm with a microplate reader. Antibody titres were determined as described above.
To measure plasma HRP concentrations, 96 well microtitre plates were coated with goat anti‐HRP antibody (Sigma) for two hours at 37°C. All subsequent steps were performed at 37°C with extensive washing between steps. Plates were blocked with 1% BSA for two hours. Diluted plasma samples in PBS‐BT were added to the wells and incubated for two hours. Plate development and measurement were as described for the OVA specific IgE.
Plasma OVA concentration was measured by sandwich ELISA using anti‐OVA IgG (Chemicon, California, USA) and HRP conjugated rabbit anti‐OVA IgG (Rockland, Pennsylvania, USA) for capture and detection antibodies, respectively, as described previously.30
Histology
Cryostat sections (5 μm) of stomach were prepared and stained with haematoxylin and periodic acid‐Schiff (PAS) reaction for detection of C albicans or with toluidine blue for identification of mast cells. The number of mast cells in the stomach was counted using a high power field in a section from each specimen. According to Nakajima and colleagues,31 with some modifications, the number of mast cells in the gastric mucosa was counted in a high power field measuring 0.65 mm2 using a light microscope equipped with a 10× objective and a 10× eyepiece containing a reticule (Olympus, Tokyo, Japan). The counting areas included stratified squamous epithelium, lamina muscularis mucosa, and submucosal layer in the forestomach, and gastric pits and fundic glands in the glandular stomach. The cell with stained material dispersed diffusely was taken as evidence of degranulated mast cell. All mast cell counts were performed by a single observer.
Reconstitution of mast cells in W/Wv mice
Mast cell deficient W/Wv mice were reconstituted with in vitro bone marrow derived mast cells (BMMC) from congenic control +/+ mice according to Kung and colleagues.32 Bone marrow from femurs was flushed out and seeded in RPMI1640 medium (GIBCO‐BRL, Tokyo, Japan) supplemented with 10 ng/ml recombinant murine interleukin 3 (PeproTech EC, London, UK), 10% heat inactivated fetal calf serum (Biological Industries, Kibbutz Beit Haemek, Israel), 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μg/ml gentamycin, at a concentration of 5×105 nucleated cells/ml, and cultured at 37°C in a humidified atmosphere containing 5% CO2. Culture medium was replaced every seven days. After five weeks of culture, cells were harvested and suspended in PBS. Staining of cells with toluidine blue indicated that nearly 99% of viable cells were mast cells. A total of 1×107 BMMC were injected intravenously into each W/Wv mouse through the tail vein. Five weeks later, the reconstituted mice were inoculated with C albicans, as described above, and then subjected to gastrointestinal permeation experiments.
Statistics
Results are presented as means (SEM). The unpaired or paired t test or Tukey‐Kramer's test following one way analysis of variance was used to compare mean values. StatView for Macintosh (version 5.0, SAS institute Inc., North Carolina, USA) was used for analysis.
Results
Sensitisation against orally administered OVA in BALB/c mice with gastrointestinal Candida colonisation
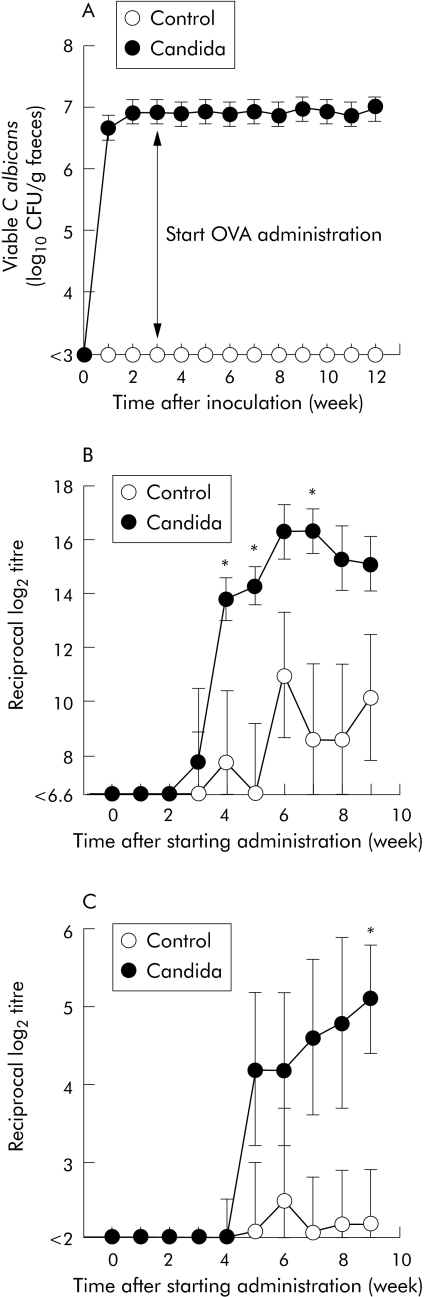
To study the effect of chronic colonisation of C albicans in the gastrointestinal tract on sensitisation against orally administered antigen, BALB/c mice were intragastrically inoculated with C albicans. By weekly counting of faecal specimens after inoculation, a high faecal recovery of C albicans was observed in all mice throughout the experimental period (fig 1A). Additionally, counting of organisms in gastrointestinal tissues by quantitative culture after euthanasia of animals revealed that colonisation occurred in the stomach, jejunum, ileum, and colon in all mice (5.5 (0.2), 1.8 (0.1), 2.3 (0.1), and 3.3 (0.0) log10 CFU/g tissue, respectively). No organism was detected in faeces or tissues of control mice without inoculation of C albicans. These data suggest that chronic colonisation of C albicans was established in the gastrointestinal tract of mice after single inoculation of C albicans.
Figure 1 Time course of changes in recovery of organisms from faeces and titres of serum antibodies specific to intragastrically administered ovalbumin (OVA) in BALB/c mice with and without gastrointestinal Candida inoculation. The number of viable C albicans in faeces (A) was determined weekly by quantitative culture method. Anti‐OVA IgG (B) and IgE (C) titres were measured in sera taken at the times indicated after starting intragastric administration of OVA. Values are means (SEM) of six mice per group. *p<0.05 compared with control mice without Candida inoculation at each time point.
Figure 1B and 1C show the time course of changes in the titre of serum antibodies against intragastrically administered OVA. In mice with gastrointestinal Candida colonisation, the anti‐OVA IgG titre began increasing at three weeks after starting administration of OVA and stabilised at six weeks (fig 1B). The anti‐OVA IgG titre tended to be higher in mice with Candida colonisation than in control mice without Candida colonisation throughout the experimental period, and there was significant difference between the two groups at 4, 5, and 7 weeks after starting OVA administration. Anti‐OVA IgE titre began increasing at five weeks after initiating OVA administration (fig 1C). As with the IgG titre, the anti‐OVA IgE titre tended to be higher in mice with Candida colonisation than in control mice, with a significant difference apparent between the groups nine weeks after initial OVA administration.
Permeation of orally administered proteins in BALB/c mice with gastrointestinal Candida colonisation
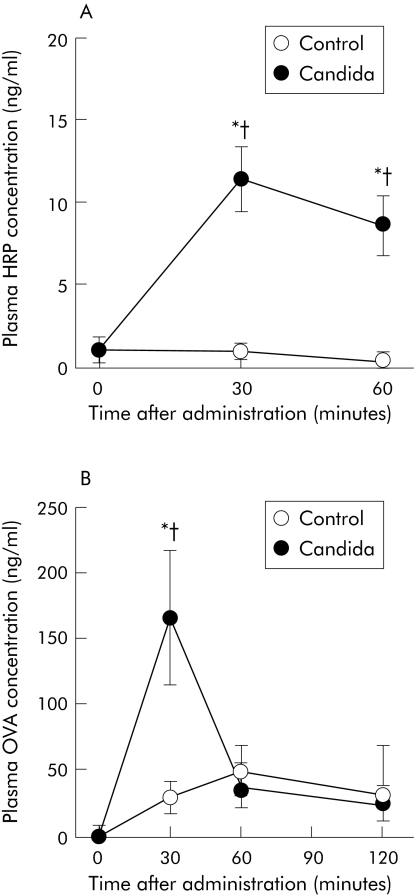
We examined gastrointestinal permeation of HRP in BALB/c mice with gastrointestinal Candida colonisation, because it is simple to detect this macromolecule by ELISA. On the day of the permeation experiment, we confirmed high faecal recovery of C albicans (6.8 (0.2) log10 CFU/g faeces) in all mice inoculated with C albicans. Figure 2A shows the time course of changes in plasma HRP concentrations in BALB/c mice after intragastric administration of this molecule. In control mice without Candida colonisation, no significant increase in plasma HRP concentrations was observed after administration. Conversely, plasma HRP concentrations in mice with gastrointestinal Candida colonisation increased significantly, peaking at 30 minutes and remaining high for 60 minutes after administration. HRP concentrations were significantly higher in mice with Candida colonisation than control mice at 30 and 60 minutes. We next examined permeation of a food antigen, OVA. As shown in fig 2B, compared with control mice, plasma OVA concentrations in mice with gastrointestinal Candida colonisation increased significantly 30 minutes after administration.
Figure 2 Time course of changes in plasma concentration of intragastrically administered proteins in BALB/c mice with and without gastrointestinal Candida colonisation. Concentrations of horseradish peroxidase (HRP) (A) and ovalbumin (OVA) (B) were measured in plasma samples of mice taken at the times indicated after intragastric administration. Values are means (SEM) of six mice per group. *p<0.05 compared with time point = 0; †p<0.05 compared with control mice at each time point.
Mast cell degranulation in gastrointestinal mucosa of BALB/c mice with gastrointestinal Candida colonisation
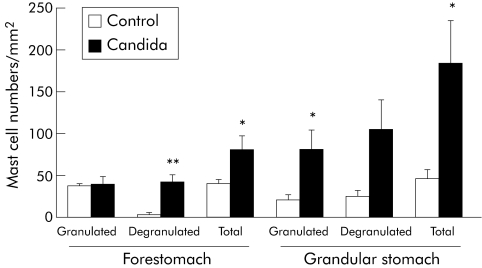
On histochemical examination of gastric mucosa in BALB/c mice killed on the day of the permeation experiment, PAS staining revealed colonisation of C albicans on the surface of the forestomach in all mice inoculated with C albicans (data not shown). Toluidine blue staining showed that a number of mast cells were infiltrated in both the forestomach and glandular stomach in Candida inoculated mice, and that degranulation was evident in the majority of cells. Quantitative examination of mast cells in histological sections of stomach in BALB/c mice is shown in fig 3. In the forestomach, numbers of granulated cells were the same in mice with Candida colonisation and control mice, whereas numbers of degranulated and total cells (that is, granulated plus degranulated) were significantly higher in mice with Candida colonisation than in control mice. In the glandular stomach, numbers of both granulated and degranulated cells were higher in mice with Candida colonisation than in control mice.
Figure 3 Number of granulated and degranulated mast cells and total number of mast cells in the forestomach and glandular stomach of mice with and without Candida inoculation. The cryostat section of stomach was stained with toluidine blue and the number of mast cells in the stomach was counted under high magnification in a section from each sample. Values are means (SEM) of six mice per group. *p<0.05; **p<0.01 compared with control mice without Candida inoculation.
Permeation of orally administered OVA in mast cell deficient mice with gastrointestinal Candida colonisation
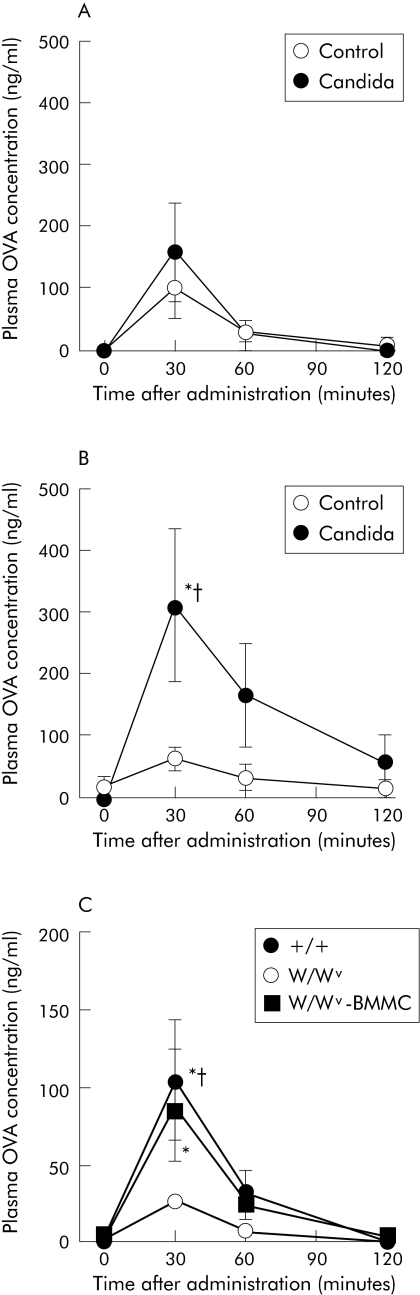
To elucidate the involvement of mast cells in increased permeation of OVA in mice with gastrointestinal Candida colonisation, we examined OVA permeation in mast cell deficient W/Wv mice. As shown in fig 4A, W/Wv mice with gastrointestinal Candida colonisation showed no significant increase in plasma OVA concentrations after administration, and levels were comparable with those in mice without Candida colonisation for the duration of the experimental period. Conversely, plasma OVA concentrations increased significantly in congenic littermate control +/+ mice with gastrointestinal Candida colonisation. Concentrations peaked at 30 minutes after administration and were significantly higher in mice with Candida colonisation than in those without Candida colonisation (fig 4B). Quantitative culture of C albicans isolated from pooled faeces for each Candida inoculated group showed that the number of organisms was higher in W/Wv mice (6.5 log10 CFU/g faeces) than in +/+ mice (5.5 log10 CFU/g faeces). No organism was detected in the faeces of control mice without inoculation of C albicans. These results indicate that the increase in OVA permeation by gastrointestinal Candida colonisation requires mast cells.
Figure 4 Time course of changes in plasma concentrations of intragastrically administered ovalbumin (OVA) in WBB6F1 mice with and without Candida inoculation. OVA concentrations were measured in plasma samples obtained at the times indicated after intragastric administration. (A, B) Results from mast cell deficient (W/Wv) and wild‐type (+/+) mice, respectively. (C) Data on +/+, W/Wv, and bone marrow derived mast cell (BMMC) transplanted W/Wv mice inoculated with C albicans. Values are means (SEM) of six mice per group. *p<0.05 compared with time = 0; †p<0.05 compared with control mice without Candida colonisation in (A) and (B) and W/Wv mice in (C), at each time point.
To confirm this finding, we then determined whether reconstitution of mast cells enhances OVA permeation in W/Wv mice with gastrointestinal Candida colonisation. As shown in fig 4C, BMMC transplanted W/Wv mice with gastrointestinal Candida colonisation as well as +/+ mice showed a significant increase in plasma OVA concentrations 30 minutes after administration, although no such significant increase was observed in W/Wv mice without BMMC transplantation. OVA concentrations at 30 minutes were significantly higher in +/+ mice than in W/Wv mice without BMMC transplantation. In BMMC transplanted W/Wv mice, OVA concentrations at 30 minutes tended to be higher than in W/Wv mice without BMMC transplantation and were comparable with those in +/+ mice. Toluidine blue staining of cryostat sections of forestomach revealed that mast cells were almost absent in W/Wv mice and that there was no significant difference in the number of granulated and degranulated mast cells between +/+ and BMMC transplanted W/Wv mice (40 (8) v 42 (7) cells/mm2 for granulated cells and 44 (8) v 45 (8) cells/mm2 for degranulated cells). In addition, quantitative culture of C albicans isolated from pooled faeces for each group on the day of the permeation experiment showed that the number of organisms was higher in W/Wv mice and BMMC transplanted W/Wv mice (7.0 and 6.8 log10 CFU/g faeces, respectively) than in +/+ mice (5.8 log10 CFU/g faeces).
Discussion
The present study showed that gastrointestinal colonisation with C albicans stimulated increased serum IgG and IgE specific to intragastrically administered OVA in BALB/c mice (fig 1), indicating therefore that gastrointestinal Candida colonisation promotes sensitisation against food antigen. Theoretically, increased permeation of food antigens in the gastrointestinal tract would elicit such immunological responses as sensitisation. Indeed, intestinal inflammation, associated with increased gut permeability, led to enhancement of sensitisation to ingested milk proteins in guinea pigs.33 In healthy humans, macromolecules such as food antigens would be excluded by the gastrointestinal mucosal barrier to prevent food allergy. However, in individuals with impaired gastrointestinal mucosal barriers, the likelihood of permeation of food antigens in an intact form would be increased. This is an important prerequisite for the development of a food allergy and may explain the prevalence of food allergy in infants and children with immature gastrointestinal mucosal barriers. In the present study, plasma concentrations of intragastrically administered proteins (that is, HRP and OVA) were significantly increased in BALB/c mice with gastrointestinal Candida colonisation (fig 2). This suggests that gastrointestinal Candida colonisation impairs the gastrointestinal mucosal barrier, which in turn enhances permeation of food antigens. Consequently, gastrointestinal colonisation by C albicans is likely to promote sensitisation against food antigens, at least in part, by affecting the gastrointestinal mucosal barrier and to increase the risk of food allergy.
We investigated the mechanism by which gastrointestinal Candida colonisation facilitates permeation of food antigens through the mucosal barrier of the gastrointestinal tract. Mast cells have been reported to be involved in the regulation of the barrier and transport properties of gastrointestinal epithelium via mediators such as rat mast cell protease II,34 tumour necrosis factor (TNF)‐α,35 and interleukin 4.36,37 In the present study, mice with gastrointestinal Candida colonisation showed an increased number of degranulated mast cells in both the forestomach and glandular stomach (fig 3). Under these conditions, mast cells should secrete mediators to increase the permeability of the gastric epithelium. In fact, despite gastric colonisation with C albicans, mast cell deficient W/Wv mice showed no overt increase in the permeation of OVA (fig 4). In addition, reconstitution of mast cells in W/Wv mice with gastrointestinal Candida colonisation enhanced OVA permeation whose levels were comparable with those in +/+ mice with Candida colonisation. The present results therefore suggest that mast cells mediate an increase in the permeation of food antigens in C albicans colonised gastrointestinal tracts. McDermott et al reported that mast cells play a critical role in increased intestinal permeability during enteric nematode infection via release of mouse mast cell protease 1 and degradation of tight junction proteins.38 This may also be the mechanism by which permeation of food antigens in C albicans colonised gastrointestinal tract is increased.
It remains unclear how C albicans colonisation stimulates mast cells. Nosal et al reported that cell wall glycoproteins from C albicans induce the release of histamine in isolated rat mast cells.39 In addition, Jouault et al showed that phospholipomannan in C albicans stimulated TNF‐α production by the mouse macrophage‐like cell line J774 via toll‐like receptor 2 (TLR2).40 Because mast cells reportedly recognise pathogenic products via TLR2,41 a ligand for TLR2 such as phospholipomannan in C albicans may elicit TNF‐α release from mast cells. We also observed that the water exudate of C albicans induces degranulation of rat basophilic leukaemia cells RBL‐2H3 in a concentration dependent fashion, suggesting degranulation of mast cells by some water soluble constituents of C albicans (unpublished data). Alternatively, anti‐C albicans antibody may stimulate mast cells. We previously observed that the titre of serum IgG1 specific to C albicans increases in BALB/c mice chronically colonised with C albicans.25 Similarly, increased anti‐C albicans IgG1 levels in the sera of mice were observed on the day of the gastrointestinal permeation experiment in the present study (data not shown). Although IgE antibodies which bind to high affinity FcεRI receptors on mast cells play a central role in mediating type I allergic responses, non‐IgE antibodies such as IgG1 could also mediate allergic responses via FcγRI receptors on mast cells.42 Therefore, it seems possible that the Candida antigen‐IgG1 complex stimulates mast cells via FcγRI receptors, which in turn enhances gastrointestinal permeability.
The present study did not elucidate the precise site at which permeation of OVA occurred in mice with gastrointestinal Candida colonisation. Although the small intestine is usually considered the primary site of uptake of food antigens,43 previous reports suggest that the stomach is a site of uptake of food antigens under some physiological conditions. Hatz et al reported that antigen challenge in rats sensitised with dinitrophenol induces degranulation of mast cells and results in increased permeation of a bystander macromolecule OVA in the stomach.44 In addition, gastric Helicobacter infection has been reported to increase gastric permeability to macromolecules.45 Therefore, it is believed that OVA permeation in mice with gastrointestinal Candida colonisation occurred in the stomach where a marked increase in the number of mast cells was observed. Further studies will be required to determine the contribution of the intestines.
Some reports suggest a positive association between Helicobacter pylori infection and the development of food allergy46,47 and AD.48 Matysiak‐Budnik et al reported that H pylori increases absorption of antigens across the gastric mucosa in mice.45 The authors also reported that Helicobacter infection inhibits the development of oral tolerance by preventing anti‐OVA IgE suppression, normally induced after OVA feeding.49 Oral tolerance is characterised by induction of a state of systemic immune non‐responsiveness towards antigens present in the gastrointestinal tract.50,51,52 This mechanism presumably prevents the development of food allergy. However, gastrointestinal pathogens, including H pylori and C albicans and/or their products, may disturb oral tolerance induction by affecting the gastrointestinal mucosal barrier and/or by their adjuvant action.49 We are currently examining whether gastrointestinal Candida colonisation influences induction of oral tolerance in the mouse model.
One may suspect that the bacterial flora in the gastrointestinal tract of Candida colonised mice may be involved in gut permeability and antibody responses to oral antigens. In our preliminary experiments using this Candida colonisation model, however, denaturing gradient gel electrophoresis of 16S rDNA amplicons showed that the bacterial composition of the stomach and faecal specimens was the same in mice with and without Candida colonisation (unpublished data). Therefore, the possibility described above could be ruled out.
In conclusion, we propose that gastrointestinal Candida colonisation promotes sensitisation against food antigens, at least partly due to mast cell mediated hyperpermeability in the gastrointestinal mucosa of mice. In contrast with this murine model however, it is not true to say that C albicans colonises the gastrointestinal tract of a healthy human. C albicans colonises the posterior dorsum of the tongue of 40–60% of healthy humans. Yeast cells dislodge from the tongue, are swallowed, and survive transit through the gastrointestinal tract, being detectable by culture of faeces. In addition, the stomach of humans lacks a non‐secretory epithelium which is the major site of Candida colonisation in the mouse forestomach. Thus it remains unclear whether the transient yeast cells in the human gut increase the permeability of the intestinal epithelium and/or affect oral immune tolerance in healthy humans. Further studies are required to prove this.
Acknowledgements
We are indebted to Dr Tatsuya Morita of Shizuoka University and Dr Taizo Nagura of Nippon Beet Sugar Mfg Co. Ltd. This work was supported in part by a grant from Uehara Memorial Foundation.
Abbreviations
AD - atopic dermatitis
BMMC - bone marrow derived mast cells
BSA - bovine serum albumin
HRP - horseradish peroxidase
OVA - ovalbumin
PAS - periodic acid‐Schiff
PBS - phosphate buffered saline
TLR - toll‐like receptor
TNF - tumour necrosis factor
Footnotes
Conflict of interest: None declared.
References
- 1.Verghese A, Prabhu K, Diamond R D.et al Synchronous bacterial and fungal septicemia. A marker for the critically ill surgical patient. Am Surg 198854276–283. [PubMed] [Google Scholar]
- 2.Bodey G P. Candidiasis in cancer patients. Am J Med 19847713–19. [PubMed] [Google Scholar]
- 3.Eras P, Goldstein M J, Sherlock P. Candida infection of the gastrointestinal tract. Medicine (Baltimore) 197251367–379. [DOI] [PubMed] [Google Scholar]
- 4.Faergemann J. Atopic dermatitis and fungi. Clin Microbiol Rev 200215545–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott B B, Jenkins D. Gastro‐oesophageal candidiasis. Gut 198223137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacour M, Zunder T, Huber R.et al The pathogenetic significance of intestinal Candida colonization—a systematic review from an interdisciplinary and environmental medical point of view. Int J Hyg Environ Health 2002205257–268. [DOI] [PubMed] [Google Scholar]
- 7.Nikkels A F, Pierard G E. Framing the future of antifungals in atopic dermatitis. Dermatology 2003206398–400. [DOI] [PubMed] [Google Scholar]
- 8.Leung D Y, Bieber T. Atopic dermatitis. Lancet 2003361151–160. [DOI] [PubMed] [Google Scholar]
- 9.Eigenmann P A, Sicherer S H, Borkowski T A.et al Prevalence of IgE‐mediated food allergy among children with atopic dermatitis. Pediatrics 1998101E8. [DOI] [PubMed] [Google Scholar]
- 10.Lever R, MacDonald C, Waugh P.et al Randomised controlled trial of advice on an egg exclusion diet in young children with atopic eczema and sensitivity to eggs. Pediatr Allergy Immunol 1998913–19. [DOI] [PubMed] [Google Scholar]
- 11.Van Reijsen F C, Felius A, Wauters E A.et al T‐cell reactivity for a peanut‐derived epitope in the skin of a young infant with atopic dermatitis. J Allergy Clin Immunol 1998101(2 Pt 1)207–209. [DOI] [PubMed] [Google Scholar]
- 12.Jackson P G, Lessof M H, Baker R W.et al Intestinal permeability in patients with eczema and food allergy. Lancet 19813171285–1286. [DOI] [PubMed] [Google Scholar]
- 13.Majamaa H, Isolauri E. Evaluation of the gut mucosal barrier: evidence for increased antigen transfer in children with atopic eczema. J Allergy Clin Immunol 199697985–990. [DOI] [PubMed] [Google Scholar]
- 14.Pike M G, Heddle R J, Boulton P.et al Increased intestinal permeability in atopic eczema. J Invest Dermatol 198686101–104. [DOI] [PubMed] [Google Scholar]
- 15.Farhadi A, Banan A, Fields J.et al Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol 200318479–497. [DOI] [PubMed] [Google Scholar]
- 16.Cenci E, Mencacci A, Spaccapelo R.et al T helper cell type 1 (Th1)‐ and Th2‐like responses are present in mice with gastric candidiasis but protective immunity is associated with Th1 development. J Infect Dis 19951711279–1288. [DOI] [PubMed] [Google Scholar]
- 17.Ekenna O, Sherertz R J. Factors affecting colonization and dissemination of Candida albicans from the gastrointestinal tract of mice. Infect Immun 1987551558–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helstrom P B, Balish E. Effect of oral tetracycline, the microbial flora, and the athymic state on gastrointestinal colonization and infection of BALB/c mice with Candida albicans. Infect Immun 197923764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy M J, Volz P A. Effects of various antibiotics on gastrointestinal colonization and dissemination by Candida albicans.J Med Vet Mycol 198523265–273. [DOI] [PubMed] [Google Scholar]
- 20.Mellado E, Cuenca‐Estrella M, Regadera J.et al Sustained gastrointestinal colonization and systemic dissemination by Candida albicans, Candida tropicalis and Candida parapsilosis in adult mice. Diagn Microbiol Infect Dis 20003821–28. [DOI] [PubMed] [Google Scholar]
- 21.Wiesner S M, Jechorek R P, Garni R M.et al Gastrointestinal colonization by Candida albicans mutant strains in antibiotic‐treated mice. Clin Diagn Lab Immunol 20018192–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Repentigny L, Phaneuf M, Mathieu L G. Gastrointestinal colonization and systemic dissemination by Candida albicans and Candida tropicalis in intact and immunocompromised mice. Infect Immun 1992604907–4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Field L H, Pope L M, Cole G T.et al Persistence and spread of Candida albicans after intragastric inoculation of infant mice. Infect Immun 198131783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy M J, Volz P A. Ecology of Candida albicans gut colonization: inhibition of Candida adhesion, colonization, and dissemination from the gastrointestinal tract by bacterial antagonism. Infect Immun 198549654–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi N, Sonoyama K, Kikuchi H.et al Gastric colonization of Candida albicans differs in mice fed commercial and purified diets. J Nutr 2005135109–115. [DOI] [PubMed] [Google Scholar]
- 26.Marshall J S, McCurdy J D, Olynych T. Toll‐like receptor‐mediated activation of mast cells: implications for allergic disease? Int Arch Allergy Immunol 200313287–97. [DOI] [PubMed] [Google Scholar]
- 27.Yu L C, Perdue M H. Role of mast cells in intestinal mucosal function: studies in models of hypersensitivity and stress. Immunol Rev 200117961–73. [DOI] [PubMed] [Google Scholar]
- 28.Reeves P G, Nielsen F H, Fahey G C., Jr AIN‐93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN‐76A rodent diet. J Nutr 19931231939–1951. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Fang C H, Hasselgren P O. Intestinal permeability is reduced and IL‐10 levels are increased in septic IL‐6 knockout mice. Am J Physiol Regulatory Integrative Comp Physiol 2001281R1013–R1023. [DOI] [PubMed] [Google Scholar]
- 30.Saitoh R, Yamaguchi N, Sonoyama K.et al Effect of enzymatic modification of dietary wheat flour for reducing its allergenicity on oral sensitization to and intestinal absorption of ovalbumin. Biosci Biotechnol Biochem 2003672483–2485. [DOI] [PubMed] [Google Scholar]
- 31.Nakajima S, Krishnan B, Ota H.et al Mast cell involvement in gastritis with or without Helicobacter pylori infection. Gastroenterology 1997113746–754. [DOI] [PubMed] [Google Scholar]
- 32.Kung T T, Stelts D, Zurcher J A.et al Mast cells modulate allergic pulmonary eosinophilia in mice. Am J Respir Cell Mol Biol 199512404–409. [DOI] [PubMed] [Google Scholar]
- 33.Fargeas M J, Theodorou V, More J.et al Boosted systemic immune and local responsiveness after intestinal inflammation in orally sensitized guinea pigs. Gastroenterology 199510953–62. [DOI] [PubMed] [Google Scholar]
- 34.Scudamore C L, Thornton E M, McMillan L.et al Release of the mucosal mast cell granule chymase, rat mast cell protease‐II, during anaphylaxis is associated with the rapid development of paracellular permeability to macromolecules in rat jejunum. J Exp Med 19951821871–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullin J M, Snock K V. Effect of tumor necrosis factor on epithelial tight junctions and transepithelial permeability. Cancer Res 1990502172–2176. [PubMed] [Google Scholar]
- 36.Berin M C, Yang P C, Ciok L.et al Role for IL‐4 in macromolecular transport across human intestinal epithelium. Am J Physiol 1999276(5 Pt 1)C1046–C1052. [DOI] [PubMed] [Google Scholar]
- 37.Colgan S P, Resnick M B, Parkos C A.et al IL‐4 directly modulates function of a model human intestinal epithelium. J Immunol 19941532122–2129. [PubMed] [Google Scholar]
- 38.McDermott J R, Bartram R E, Knight P A.et al Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci USA 20031007761–7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nosal R, Novotny J, Sikl D. The effect of glycoprotein from Candida albicans on isolated rat mast cells. Toxicon 197412103–108. [DOI] [PubMed] [Google Scholar]
- 40.Jouault T, Ibata‐Ombetta S, Takeuchi O.et al Candida albicans phospholipomannan is sensed through toll‐like receptors. J Infect Dis 2003188165–172. [DOI] [PubMed] [Google Scholar]
- 41.Varadaradjalou S, Feger F, Thieblemont N.et al Toll‐like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur J Immunol 200333899–906. [DOI] [PubMed] [Google Scholar]
- 42.Okayama Y, Kirshenbaum A S, Metcalfe D D. Expression of a functional high‐affinity IgG receptor, FcγRI, on human mast cells: Up‐regulation by IFN‐γ. J Immunol 20001644332–4339. [DOI] [PubMed] [Google Scholar]
- 43.Walker W A, Isselbacher K J, Bloch K J. Intestinal uptake of macromolecules: effect of oral immunization. Science 1972177608–610. [DOI] [PubMed] [Google Scholar]
- 44.Hatz R A, Bloch K J, Harmatz P R.et al Divalent hapten‐induced intestinal anaphylaxis in the mouse enhances macromolecular uptake from the stomach. Gastroenterology 199098894–900. [DOI] [PubMed] [Google Scholar]
- 45.Matysiak‐Budnik T, Hashimoto K, Heyman M.et al Antral gastric permeability to antigens in mice is altered by infection with Helicobacter felis. Eur J Gastroenterol Hepatol 1999111371–1377. [DOI] [PubMed] [Google Scholar]
- 46.Corrado G, Luzzi I, Lucarelli S.et al Positive association between Helicobacter pylori infection and food allergy in children. Scand J Gastroenterol 1998331135–1139. [DOI] [PubMed] [Google Scholar]
- 47.Figura N, Perrone A, Gennari C.et al Food allergy and Helicobacter pylori infection. Ital J Gastroenterol Hepatol 199931186–191. [PubMed] [Google Scholar]
- 48.Murakami K, Fujioka T, Nishizono A.et al Atopic dermatitis successfully treated by eradication of Helicobacter pylori. J Gastroenterol 19963177–82. [PubMed] [Google Scholar]
- 49.Matysiak‐Budnik T, van Niel G, Megraud F.et al Gastric Helicobacter infection inhibits development of oral tolerance to food antigens in mice. Infect Immun 2003715219–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiner H L. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol Today 199718335–343. [DOI] [PubMed] [Google Scholar]
- 51.Strobel S, Mowat A M. Immune responses to dietary antigens: oral tolerance. Immunol Today 198819173–181. [DOI] [PubMed] [Google Scholar]
- 52.Brandtzaeg P. Mechanisms of gastrointestinal reactions to food. Environ Toxicol Pharmacol 199749–24. [DOI] [PubMed] [Google Scholar]