Abstract
Background
Idiopathic slow transit constipation is one of the most severe and often intractable forms of constipation. As motor abnormalities are thought to play an important pathogenetic role, studies have been performed on the colonic neuroenteric system, which rules the motor aspects of the viscus.
Aims
We hypothesised that important neuropathological abnormalities of the large bowel are present, that these are not confined to the interstitial cells of Cajal and ganglion cells, and that the previously described reduction of enteric neurones, if confirmed, might be related to an increase in programmed cell death (apoptosis).
Patients and methods
Surgical specimens from 26 severely constipated patients were assessed by conventional and immunohistochemical methods. Specific staining for enteric neurones, glial cells, interstitial cells of Cajal, and fibroblast‐like cells associated with the latter were used. In addition, gangliar cell apoptosis was evaluated by means of indirect and direct techniques. Data from patients were compared with those obtained in 10 controls.
Results
Severely constipated patients displayed a significant decrease in enteric gangliar cells, glial cells, and interstitial cells of Cajal. Fibroblast‐like cells associated with the latter did not differ significantly between patients and controls. Patients had significantly more apoptotic enteric neurones than controls.
Conclusion
Severely constipated patients have important neuroenteric abnormalities, not confined to gangliar cells and interstitial cells of Cajal. The reduction of enteric neurones may in part be due to increased apoptotic phenomena.
Keywords: apoptosis, enteric neurones, glial cells, interstitial cells of Cajal, slow transit constipation
Chronic constipation is a symptom encountered frequently in clinical practice, and may affect a considerable percentage of the general population.1 Several mechanisms contribute to constipation, although the main two subgroups are related to obstructed defecation and slow transit.2 In particular, slow transit constipation (STC) usually represents the subgroup of patients with most severe symptoms who tend to be less influenced by therapeutic measures.3 These patients are frequently labelled as intractable,4 and the most severe ones (up to true colonic inertia) are often referred for a surgical approach.5,6
The pathophysiological causes of STC are still poorly understood, and abnormal colonic motility is thought to play an important role.7,8 Several qualitative and quantitative changes in the enteric nervous system of such patients have been described. They include abnormal enteric neurochemistry,9,10,11,12 decreased argyrophilic neurones,13 decreased intraganglionic neurofilaments,14 and hypoganglionosis of the myenteric plexus.15 More recently, a decrease in colonic interstitial cells of Cajal (ICC) in STC patients has also been reported.16,17,18
However, most of the above reports involved small groups of patients with different clinical and instrumental characteristics, and the studies usually focused on only one or two issues related to the myenteric plexus.
Here we report a neuropathological investigation of the colon in a relatively large and homogeneous group of STC patients. In particular, we wished to test the hypothesis that the abnormalities are not confined to the ICC and ganglion cells, and that the previously described reduction of enteric neurones, if confirmed, might be related to an increase in programmed cell death (apoptosis).
Patients and methods
Patients
Twenty six STC patients (25 women, one man; age range 24–78 years) undergoing colectomy with ileorectostomy for severe intractable constipation were enrolled in the study. Inclusion criteria were: (1) longstanding history of constipation (more than three years; mean 14 (range 3–59)); symptoms arose in childhood in one patient and in later life in the others; (2) one or fewer evacuations per week; (3) absence of frequent (more than two episodes per month) or chronic abdominal pain; (4) sensation of incomplete evacuation in >1/4 defecations; (5) negative history for (sub)occlusive episodes; and (6) unresponsiveness to appropriate and intensive medical treatment, including high fibre diet, stimulant and osmotic laxatives, and enemas. Intestinal transit time, measured by means of radiopaque markers, was delayed in all patients (up to more than 240 hours). Causes of secondary constipation were excluded by drug history, physical examination, and laboratory screening (blood chemistry, thyroid hormones and, where appropriate, oral glucose tolerance test, sex hormone profiles, and antinuclear antibodies). To exclude organic diseases or mechanical causes of constipation and megacolon or megarectum, each patient underwent double contrast barium enema and/or colonoscopy. Absence of Hirschsprung's disease was demonstrated by normal relaxation of the internal anal sphincter at anorectal manometry.19 No patient had evidence of obstructed defecation, as documented by anorectal manometry and/or defecography.
Controls
Ten patients (nine women, one man; age range 43–75 years) undergoing left hemicolectomy for non‐obstructing colorectal cancer were used as controls as there is evidence that the distribution of ICC is relatively uniform throughout the human colon.16 No data are available on the regional density of enteric neurones and glial cells in humans although in preliminary observations we did not detect significant regional differences between the various colonic segments, except in the rectum (G Bassotti and V Villanacci, personal observations). Control specimens were taken at least 5 cm from the resection margin in tumour free areas.
Methods
After removal, surgical specimens were immediately fixed in 10% neutral buffered formalin for 24 hours, and then 12–20 full thickness samples from the whole resected colon were taken and transversal sections obtained. For conventional histology, 5 µm paraffin sections were stained with haematoxylin‐eosin, periodic acid‐Schiff (PAS), and trichrome stain.
Immunohistochemistry
At least 40 slides for each patient were processed for immunohistochemistry. To evaluate markers of the enteric nervous system we used monoclonal antibodies towards neurone specific enolase (NSE, NCL‐NSE2, dilution 1:50; Novocastra Laboratories, Newcastle upon Tyne, UK) acting as a marker of gangliar cells, and the glial marker protein S100 (S‐100, dilution 1:50; Dako, Carpinteria, California, USA) was used.20,21 As ICC express Kit,22 an anti‐Kit antibody (rabbit polyclonal antibody, IgG, dilution 1:50; Dako) was used to detect these cells, as previously reported.23 Moreover, CD34 staining (CD34 clone QBEnd/10, dilution 1:30; Neo markers, Union City, California, USA) was used to evaluate the population of fibroblast‐like cells which are intimately associated with the ICC.24 Two methods were used as markers for apoptosis in the enteric nervous system: (a) expression of Bcl‐2 protein (BCL2 oncoprotein clone 124, dilution 1:10; DBS, Pleasantown, Australia), a proto‐oncogene responsible for specific suppression of apoptosis in several important situations,25 and well displayed in human enteric neurones,26,27 and (b) monoclonal antibody to single stranded DNA,28 using the formamide monoclonal antibody (formamide‐MAb) method (Mab F7‐26 BMS 156; Bender MedSystem, Vienna, Austria) which detects apoptotic cells in tissue processed with routine histological techniques and allows discrimination of apoptosis from necrosis.29
NSE, S‐100, CD34, and Bcl‐2 immunostaining was carried out using a peroxidase based visualisation kit (Dako LSAB) following the manufacturer's recommendations. Diaminobenzidine tetrahydrochloride was used as chromogen. Slides were then counterstained with Mayer's haematoxylin for five seconds, dehydrated, and mounted in Clarion (Biomeda, Foster City, California, USA). To account for non‐specific staining, peptides that blocked polyclonal antibody bindings (passage with normal goat serum) were used, or sections were incubated in the absence of primary antibody. In these cases, no immunostaining was detected. For Bcl‐2, expression in mucosal lymphoid cells served as an internal control.
Expression of Kit
Consecutive formalin fixed paraffin sections were dewaxed and rehydrated through decreasing alcohol series up to distilled water. Sections were then subjected to heat induced epitope retrieval by immersion in a heat resistant container filled with citrate buffer solution (pH 6.0) placed in a pressure cooker and microwaved for 20 minutes. Endogenous peroxidase activity was suppressed by incubation with 3% solution of H2O2 for five minutes. Kit immunostaining was carried out using a peroxidase based visualisation kit (Dako EnVision) following the manufacturer's recommendations. Kit positive mast cells served as an internal control.
Anti single stranded DNA immunohistochemistry
Sections 2–3 μm thick were warmed overnight at 60°C, then dewaxed and rehydrated through decreasing alcohol series up to distilled water. Thereafter, sections were incubated for five minutes in phosphate buffered saline with addition of 20% Tween 20, followed by passage with proteinase K (Dako) for 20 minutes. Sections were then rinsed with distilled water and heated in 50% formamide prewarmed to 60°C for 20 minutes. After cooling, endogenous peroxidase activity was suppressed by incubation with a 3% solution of H2O2 for five minutes. Normal serum diluted 1:50 was applied for 10 minutes at room temperature, followed by anti‐DNA MAb for 30 minutes, according to the manufacturer's recommendations. After that, sections were incubated at room temperature with secondary polymeric antibody for 20 minutes and ABC (Kit super sensitive non‐biotin detection system; Menarini, Firenze, Italy) for 30 minutes. Finally, a five minute reaction in the dark with diaminobenzidine (Bio‐Optica, Milano, Italy) was carried out, and sections were then counterstained with Mayer's haematoxylin for five seconds, dehydrated, and mounted in Clarion (Biomeda). Positivity was observed under the microscope as an intense brown reaction.
The presence of lymphocytes was assessed by means of a monoclonal mouse antihuman CD3 antibody (dilution 1:40; Dako Cytomation).
Colonic smooth muscle was evaluated by means of an anti‐α‐actin monoclonal antibody (dilution 1:100; Biogenex, San Ramon, California, USA).
Data analysis
All slides were coded and analysed blind by two pathologists. For NSE, S100, and CD3, as well as Bcl‐2 and formamide‐MAb positive cells, both the submucosa and myenteric plexuses were taken into account by optical microscopy at ×20 magnification (Olympus BX 40). For each patient, the number of immunopositive cells was calculated and expressed as the mean of cells on 10 well stained and well oriented microscopic fields for each region of interest. To be considered positive, the intensity of cell immunostaining in relation to possible background had to be from moderate to strong, as described previously.30
The density of ICC was graded, according to a previously described method,31,32 after evaluation of 10 well stained and well oriented fields at ×20 magnification. The three previously identified populations of ICC were taken into consideration33,34: IC‐SM, along the submucosal surface of the circular muscle bundle; IC‐MY, within the intermuscular space between circular and longitudinal muscle layers (myenteric region, which displays the highest yield of ICC in normal tissue16,18,31); and IC‐IM, within the muscle fibres of the circular and longitudinal muscle layers. Nucleated cells as well as Kit positive labelled elongated structures were considered for analysis.32
For CD34, the strength of immunostaining (graded as either present or severely depleted/absent, according to recently reported criteria35) was calculated around the myenteric plexus, between the elements of the plexus, within the longitudinal and circular muscle elements. Care was taken not to include vessels in the evaluation; however, the effectiveness of CD34 staining was indicated by the staining of capillaries in subjects with severe depletion/absence in other locations.35
Statistical analysis
The Komolgorov‐Smirnov test for normality was applied and showed the data to be normally distributed. Data from controls and patients were thus compared using the Student's t test for unpaired data (two tailed) and Pearson's correlation coefficient, where appropriate. Values of p<0.05 were chosen for rejection of the null hypothesis. Data are expressed as means (95% confidence interval).
Results
Conventional histology
In both groups, the mucosa, submucosa, smooth muscle, and nerve plexus architecture appeared normal on haematoxylin‐eosin, trichrome, and PAS staining. No inflammatory cells (or intranuclear or viral inclusions) were observed in or around muscular or nervous structures.
No patient exhibited hyperplastic changes (for example, giant ganglia) of the submucosal plexus, thereby excluding a diagnosis of intestinal neuronal dysplasia.36 Moreover, no inclusion body myopathy was found in smooth muscle.37 The presence of (pseudo) melanosis coli was shown in 80% of patients; those with a history of anthraquinone laxative use.
Immunohistochemistry
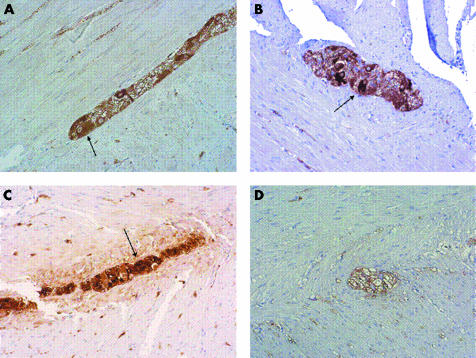
NSE expression (fig 1A, B) was significantly decreased in STC patients compared with controls, in both the myenteric (28.5 (24–33) v 64.4 (55–73) cells; p<0.001) and submucosal (21 (19–24) v 58 (46–70) cells; p<0.001) plexus. Similar results were detected for S100 expression (fig 1C, D), again significantly decreased in STC patients compared with controls in both the myenteric (174 (156–191) v 214 (190–238) cells, p = 0.021) and submucosal (97 (85–109) v 127 (94–161) cells; p = 0.026) plexus.
Figure 1 Neurone specific enolase expression in the myenteric plexus in a control subject (A) and in a patient with slow transit constipation (B). The latter showed a decreased number of gangliar cells. Original magnification ×20. Arrows indicate gangliar cells. S100 expression in a control subject (C) and in a patient (D), with the latter showing a reduction in glial cells. Original magnification ×20. Arrow indicates glial cells.
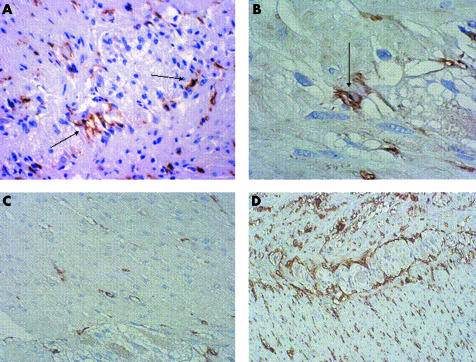
With regard to ICC (fig 2A–C), a significant decrease was found in patients for IC‐MY (141.3 (124–159) v 214 (154–274) cells; p = 0.0017) and IC‐SM (14 (11–17) v 29 (23–35) cells; p<0.001) but not for IC‐IM (36 (30–42) v 38 (32–44) cells; p = 0.68). Scattered Kit positive mast cells were observed in patient mucosa, and were numerically distributed as in control tissue.
Figure 2 CD117 expression in a control subject (A, B) and in a patient with slow transit constipation (C). Note the decrease in interstitial cells of Cajal (ICC) in the patient's tissue. Original magnifications ×40 (A, C) and ×100 (B). ICC are indicated by arrows. (D) CD34 expression in a patient. Original magnification ×20.
No relationship was found between the number of myenteric neurones or ICC‐MY and duration of constipation (for myenteric neurones r = −0.02, p = 0.89; for ICC‐MY r = 0.156, p = 0.44).
No differences between the groups were found in expression of CD34 (fig 2D), which was severely depleted/absent in two patients and two controls (χ2 test, p = 0.66).
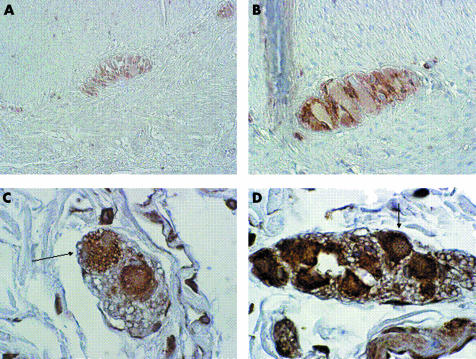
Expression of Bcl‐2 (fig 3A, B) was significantly decreased in STC patients compared with controls, in both the myenteric (124 (108–141) v 167 (127–207) cells; p = 0.015) and submucosal (30 (26–33) v 55.4 (47–64) cells; p<0.001) plexus. Expression of Bcl‐2 in mucosal lymphoid cells of patients was comparable with that found in controls.
Figure 3 Bcl2 expression in a control subject (A, original magnification ×20) and in a patient with slow transit constipation (B, original magnification ×40). Apoptotic neurones (arrows) in a control subject (C) and in a patient (D). Original magnification ×40.
The number of apoptotic enteric neurones (fig 3C, D) was significantly increased in the myenteric plexus of STC patients (18 (15–20) v 11 (7–14) cells; p = 0.0052) whereas no differences were found in the submucosal plexus (16 (12–19) v 12 (9–15) cells; p = 0.15).
No lymphocytic infiltration (assessed by CD3) was observed in either the submucosal or myenteric plexus of patients or controls.
All patients and controls showed strong intensity for α‐actin immunostaining so that colonic smooth muscle was judged to display normal characteristics.
Discussion
This study, carried out in a homogeneous and relatively large group of patients with severe and intractable STC, showed that abnormalities of the enteric nervous system are present in such patients but that they are not confined to neurones and ICC. Although a significant decrease in enteric neurones and ICC was found in patients compared with the control group, a further significant decrease was discovered in patients concerning glial cells, which were reduced in both the myenteric and submucosal plexus. We consider this finding interesting and worthy of comment.
Enteric glial cells originate from the neural crest and provide support for neuronal elements.38 The best known function of the glia in the adult is the formation of myelin sheaths around axons, thus allowing the fast connections essential for nervous system function. The glia also maintains appropriate concentrations of ions and neurotransmitters in the neuronal environment. An increasing body of evidence indicates that glial cells are essential regulators of the formation, maintenance, and function of synapses, the key functional units of the nervous system.39 Thus as enteric glial cells are thought to act as intermediaries in enteric neurotransmission,40 their decrease might further weaken the already precarious neuroenteric balance due to the decrease in neuronal elements and ICC found in STC patients (see below). We are as yet unable to explain this decrease in glial elements in our patients, and there is no literature support, except for the reduction of these cells found in aged rats.41 However, no such reduction was detected in the similarly aged control group; it therefore appears unlikely that the decrease was due to aging, at least in the proportions we found.
Our patients presented with a significant drop in ICC in the myenteric and submucosal plexus. This finding is consistent with recent studies16,17,18 which also detected decreased ICC volume in STC patients.42 The role of ICC as intestinal pacemakers has been clearly established in experimental animal models, which have shown that a lack of ICC networks leads to absence of slow waves and is accompanied by delayed or absent intestinal motility.43,44 Therefore, reduction or loss of ICC function might decrease or eliminate colonic electrical slow wave activity, thereby reducing the contractile response and resulting in delayed transit in STC patients. How can this finding be explained? Very recent evidence has shown that expression of c‐kit mRNA and c‐kit protein was significantly decreased in STC patients, suggesting that alterations in the c‐kit signal pathway may play an important role in ICC reduction in such patients.45
The reduction in ICC was not accompanied by loss of CD34 positive fibroblasts, as observed in other pathological conditions.35 In a mouse model, blockage of Kit receptors caused transdifferentiation of intestinal ICC to a smooth muscle phenotype46; it is tempting to speculate that this inherent plasticity between ICC and smooth muscle cells might also occur in the human colon. If verified, this hypothesis could be exploited because, if ICC do not die in STC but rather redifferentiate, it may be possible to create conditions that would shift the phenotype back towards ICC.
A third important neuropathological aspect is loss of enteric neural elements found in these patients. Similar findings have previously been reported in small groups of STC patients15,17 although not consistently.47 As the mechanisms that lead to depletion of enteric neurones are unknown, we tested the hypothesis that neuronal loss might be due to an increase in programmed cell death (that is, an apoptotic phenomenon).
Therefore, we first obtained indirect evidence by assessing Bcl‐2, a unique proto‐oncogene localised to mitochondria and able to block apoptosis in a variety of in vitro and in vivo situations, suggesting interference with a central mechanism of apoptosis.48,49 Expression of Bcl‐2 was significantly lower in the enteric neural elements of STC patients compared with controls, in both the myenteric and submucosal plexus, thus suggesting impairment of antiapoptotic factors.
Then we assessed apoptosis directly in enteric neurones by means of the formamide‐MAb method, a technique unaffected by DNA breaks and able to identify apoptotic cells and to discriminate between them and necrotic cells.29 We found that the number of apoptotic enteric neurones in patients was significantly increased in the myenteric (but not in the submucosal) plexus, suggesting that apoptosis probably plays a role in loss of these cells. It is also worth noting that there is some literature evidence suggesting that, at least in colonic epithelia, melanosis coli may be a non‐specific marker of increased apoptosis.50 These data did not however include submucosal or myenteric plexus evaluation.
Neuronal depletion further supports a role of the (deranged) enteric nervous system in the pathophysiology of STC. We feel that the overall findings from this study all point towards a synergic effect of each abnormality in causing abnormal colonic motor activity and, therefore, symptoms complained of by patients. A decrease in ICC impairs pacemaker activity, whereas loss of enteric glial cells and neurones reduces the nervous stimuli to effector (smooth muscle) cells, which are therefore unable to yield an effective and coordinated force able to carry out the main physiological purposes of the viscus, namely mixing, storage, and expulsion of the contents. The fact that the above abnormalities are incomplete (that is, there was not complete loss of any of the anatomical elements evaluated) may explain why residual colonic motor activity may be still detected in very severely constipated patients.5,51
The causes of the abnormalities we documented are still unclear, and we have not found results supporting proposed pathophysiological mechanisms in late onset STC,52 such as lymphocytic epithelioganglionitis53 and localisation of neurotropic viruses in the myenteric plexus.7
The study does have some limitations. For instance, the choice of controls and the (transverse) sectioning technique could be considered suboptimal. However, at least for children, there is some evidence that colonic transverse sections yield similar counts of neuronal density compared with longitudinal sections.54
In conclusion, patients with intractable “idiopathic” STC display important neuropathological enteric abnormalities, which are not confined to ICC and neuronal elements. Loss of the latter may be due in part to increased apoptosis, and this observation may be of some interest in the light of future therapeutic approaches aiming to reduce this phenomenon. Lastly, ever increasing knowledge of the basic mechanisms of this entity might in the future help to delete “idiopathic” from the definition of this form of constipation.
Abbreviations
STC - slow transit constipation
ICC - interstitial cells of Cajal
NSE - neurone specific enolase
MAb - monoclonal antibody
PAS - periodic acid‐Schiff
Footnotes
Conflict of interest: None declared.
References
- 1.Stewart W F, Liberman J N, Sandler R S.et al Epidemiology of constipation (EPOC) study in the United States: relation of clinical subtypes to socioeconomic features. Am J Gastroenterol 1999943530–3539. [DOI] [PubMed] [Google Scholar]
- 2.Lembo A, Camilleri M. Chronic constipation. N Engl J Med 20033491360–1368. [DOI] [PubMed] [Google Scholar]
- 3.Schiller L R. Review article: the therapy of constipation. Aliment Pharmacol Ther 200115749–763. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M, Thompson W G, Fleshman J W.et al Clinical management of intractable constipation. Ann Intern Med 1994121520–528. [DOI] [PubMed] [Google Scholar]
- 5.Bassotti G, Chistolini F, Sietchiping Nzepa F.et al Colonic propulsive impairment in intractable slow‐transit constipation. Arch Surg 20031381302–1304. [DOI] [PubMed] [Google Scholar]
- 6.Bassotti G, de Roberto G, Sediari L.et al Toward a definition of colonic inertia. World J Gastroenterol 2004102465–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knowles C H, Martin J E. Slow transit constipation: a model of human gut dysmotility. Review of possible aetiologies. Neurogastroenterol Motil 200012181–196. [DOI] [PubMed] [Google Scholar]
- 8.Bassotti G, Iantorno G, Fiorella S.et al Colonic motility in man: features in normal subjects and in patients with chronic idiopathic constipation. Am J Gastroenterol 1999941760–1770. [DOI] [PubMed] [Google Scholar]
- 9.Koch T, Carney J A, Go V L. Idiopathic chronic constipation is associated with decreased colonic vasoactive intestinal peptide. Gastroenterology 198894300–310. [DOI] [PubMed] [Google Scholar]
- 10.Sjolund K, Fasth S, Ekman R.et al Neuropeptides in idiopathic chronic constipation (slow transit constipation). Neurogastroenterol Motil 19979143–150. [DOI] [PubMed] [Google Scholar]
- 11.Faussone‐Pellegrini M S, Infantino A, Matini P.et al Neuronal anomalies and normal muscle morphology at the hypomotile ileocecocolonic region of patients affected by idiopathic chronic constipation. Histol Histopathol 1999141119–1134. [DOI] [PubMed] [Google Scholar]
- 12.Zhao R H, Baig M K, Mack J.et al Altered serotonin immunoreactivities in the left colon of patients with colonic inertia. Colorectal Dis 2002456–60. [DOI] [PubMed] [Google Scholar]
- 13.Krishnamurti S, Schuffler M D, Rohrmann C A.et al Severe idiopathic constipation is associated with a distinctive abnormality of the colonic myenteric plexus. Gastroenterology 19858826–34. [DOI] [PubMed] [Google Scholar]
- 14.Schouten W R, ten Kate F J, de Graaf E J.et al Visceral neuropathy in slow transit constipation: an immunohistochemical investigation with monoclonal antibodies against neurofilament. Dis Colon Rectum 1993361112–1117. [DOI] [PubMed] [Google Scholar]
- 15.Wedel T, Roblick U J, Ott V.et al Oligoneural hypoganglionosis in patients with idiopathic slow transit constipation. Dis Colon Rectum 20024554–62. [DOI] [PubMed] [Google Scholar]
- 16.Lyford G L, He C L, Soffer E.et al Pan‐colonic decrease in interstitial cells of Cajal in patients with slow transit constipation. Gut 200251496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wedel T, Spiegler J, Soellner S.et al Enteric nerves and interstitial cells of Cajal are altered in patients with slow transit constipation and megacolon. Gastroenterology 20021231459–1467. [DOI] [PubMed] [Google Scholar]
- 18.Tong W D, Liu B H, Zhang L Y.et al Decreased interstitial cells of Cajal in the sigmoid colon of patients with slow transit constipation. Int J Colorect Dis 200419467–473. [DOI] [PubMed] [Google Scholar]
- 19.Phillips S F, Pemberton J H. Megacolon: congenital and acquired. In: Feldman M, Scharschmidt BF, Sleisenger MH. Gastrointestinal and liver disease, 6th edn. Philadelphia: WB Saunders Company, 19981810–1819.
- 20.Krammer H J, Karahan S T, Sigge W.et al Immunohistochemistry of markers of the enteric nervous system in whole‐mount preparations of the human colon. Eur J Pediatr Surg 19944274–278. [DOI] [PubMed] [Google Scholar]
- 21.Dzienis‐Koronkiewicz E, Debek W, Sulkowska M.et al Suitability of selected markers for identification of elements of the intestinal nervous system (INS). Eur J Pediatr Surg 200212397–401. [DOI] [PubMed] [Google Scholar]
- 22.Williams D E, Eisenman J, Baird A.et al Identification of a ligand for the c‐kit proto‐oncogene. Cell 199063167–174. [DOI] [PubMed] [Google Scholar]
- 23.Horisawa M, Watanabe Y, Torihashi S. Distribution of c‐Kit immunopositive cells in normal human colon and in Hirschsprung's disease. J Pediatr Surg 1998331209–1214. [DOI] [PubMed] [Google Scholar]
- 24.Vanderwinden J M, Liu H, De Laet M H.et al CD34+ cells in human intestine are fibroblasts adjacent to, but distinct from interstitial cells of Cajal. Lab Invest 19997959–65. [PubMed] [Google Scholar]
- 25.Adams J M, Cory S. The Bcl‐2 protein family: arbiters of cell survival. Science 19982811322–1326. [DOI] [PubMed] [Google Scholar]
- 26.Wester T, Olsson Y, Olsen L. Expression of bcl‐2 in enteric neurons in normal human bowel and in Hirschsprung disease. Arch Pathol Lab Med 19991231264–1268. [DOI] [PubMed] [Google Scholar]
- 27.De Giorgio R, Santini D, Ceccarelli C.et al Defective expression of Bcl‐2 in the enteric nervous system (ENS): a new potentially useful neuro‐pathological marker for severe functional bowel disorders (FBD). Ital J Gastroenterol 199628(suppl 2)100 [Google Scholar]
- 28.Frankfurt O S, Robb J A, Sugarbaker E V.et al Monoclonal antibody to single‐stranded DNA is a specific and sensitive cellular marker of apoptosis. Exp Cell Res 1996226387–397. [DOI] [PubMed] [Google Scholar]
- 29.Frankfurt O S, Krishan A. Identification of apoptotic cells by formamide‐induced DNA denaturation in condensed chromatin. J Histochem Cytochem 200149369–378. [DOI] [PubMed] [Google Scholar]
- 30.Maurer C A, Friess H, Buhler S S.et al Apoptosis inhibiting factor Bcl‐XL might be the crucial member of the Bcl‐2 gene family in colorectal cancer. Dig Dis Sci 1988432641–2648. [DOI] [PubMed] [Google Scholar]
- 31.Hagger R, Gharaie S, Finlayson C.et al Regional and transmural density of interstitial cells of Cajal in human colon and rectum. Am J Physiol 199838G1309–G1316. [DOI] [PubMed] [Google Scholar]
- 32.Bassotti G, Battaglia E, Bellone G.et al Interstitial cells of Cajal, enteric nerves and glial cells in colonic diverticular disease. J Clin Pathol 200558973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders K M. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology 1996111492–515. [DOI] [PubMed] [Google Scholar]
- 34.Ward S M, Sanders K M, Hirst G D. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol Motil 200416(suppl 1)112–117. [DOI] [PubMed] [Google Scholar]
- 35.Streutker C J, Huizinga J D, Campbell F.et al Loss of CD117 (c‐kit)‐ and CD34‐positive ICC and associated CD34‐positive fibroblasts defines a subpopulation of chronic intestinal pseudo‐obstruction. Am J Surg Pathol 200327228–235. [DOI] [PubMed] [Google Scholar]
- 36.Stoss F, Meier‐Ruge W. Experience with neuronal intestinal dysplasia (NID) in adults. Eur J Pediatr Surg 19944298–302. [DOI] [PubMed] [Google Scholar]
- 37.Knowles C H, Nickols C D, Scott S M.et al Smooth muscle inclusion bodies in slow transit constipation. J Pathol 2001193390–397. [DOI] [PubMed] [Google Scholar]
- 38.Gershon M D, Rothman T P. Enteric glia. Glia 19914195–204. [DOI] [PubMed] [Google Scholar]
- 39.Jessen K R. Glial cells. Int J Biochem Cell Biol 2004361861–1867. [DOI] [PubMed] [Google Scholar]
- 40.Ruhl A, Nasser Y, Sharkey K A. Enteric glia. Neurogastroenterol Motil 200416(suppl 1)44–49. [DOI] [PubMed] [Google Scholar]
- 41.Phillips R J, Kieffer E J, Powley T L. Loss of glia and neurons in the myenteric plexus of the aged Fischer 344 rat. Anat Embryol (Berl) 200420919–30. [DOI] [PubMed] [Google Scholar]
- 42.He C L, Burgart L, Wang L.et al Decreased interstitial cell of Cajal volume in patients with slow‐transit constipation. Gastroenterology 200011814–21. [DOI] [PubMed] [Google Scholar]
- 43.Ward S M, Burns A J, Torihashi S.et al Mutation of the proto‐oncogene c‐kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol (Lond) 199448091–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huizinga J D, Thuneberg L, Kluppel M.et al W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 1995373347–349. [DOI] [PubMed] [Google Scholar]
- 45.Tong W D, Liu B H, Zhang L Y.et al Expression of c‐kit messenger ribonucleic acid and c‐kit protein in sigmoid colon of patients with slow transit constipation. Int J Colorect Dis 200520367–367. [DOI] [PubMed] [Google Scholar]
- 46.Torihashi S, Nishi K, Tokutomi Y.et al Blockade of kit signaling induces transdifferentiation of interstitial cells of Cajal to a smooth muscle phenotype. Gastroenterology 1999117140–148. [DOI] [PubMed] [Google Scholar]
- 47.Park H J, Kamm M A, Abbasi A M.et al Immunohistochemical study of the colonic muscle and innervation in idiopathic chronic constipation. Dis Colon Rectum 199538509–513. [DOI] [PubMed] [Google Scholar]
- 48.Korsmeyer S J. Bcl‐2: an antidote to programmed cell death. Cancer Surv 199215105–108. [PubMed] [Google Scholar]
- 49.Hockenbery D M. The bcl‐2 oncogene and apoptosis. Semin Immunol 19924413–420. [PubMed] [Google Scholar]
- 50.Byers R J, Marsh P, Parkinson D.et al Melanosis coli is associated with an increase in colonic epithelial apoptosis and not with laxative use. Histopathology 199730160–164. [DOI] [PubMed] [Google Scholar]
- 51.Bassotti G, Chiarioni G, Germani U.et al Endoluminal instillation of bisacodyl in patients with severe (slow transit type) constipation is useful to test residual colonic propulsive activity. Digestion 19996069–73. [DOI] [PubMed] [Google Scholar]
- 52.Knowles C H, Scott S M, Lunniss P J. Slow transit constipation. A disorder of pelvic autonomic nerves? Dig Dis Sci 200146389–401. [DOI] [PubMed] [Google Scholar]
- 53.Lindberg G, Glia A, Nyberg G.et al Lymphocytic epithelioganglionitis—a new entity causing severe motility disorders of the gut. Gastroenterology 1999116G4476 [Google Scholar]
- 54.Smith V V. Intestinal neuronal density in childhood: a baseline for the objective assessment of hypo‐ and hyperganglionosis. Pediatr Pathol 199313225–237. [DOI] [PubMed] [Google Scholar]