Abstract
Background
Recently, non‐invasive techniques such as 3 dimensional ultrasonography (3DUS) have been developed to assess gastric wall characteristics and to investigate their relationship with upper gastrointestinal sensations. To date, no head‐to‐head comparison has been carried out between the barostat and the 3DUS technique.
Aim
To compare barostat and 3DUS and to investigate the relationship between gastric volumes and sensations in patients with functional dyspepsia and in healthy subjects.
Patients and methods
Gastric accommodation was studied in 15 patients with functional dyspepsia and in 15 healthy subjects after ingestion of a liquid nutrient (300 kcal) using barostat and 3DUS in random order for 60 min. Proximal gastric relaxation was measured using barostat and gastric volume using 3DUS. Change in gastric volume, acquired by 3DUS, was expressed as total gastric volume/proximal volume ratio (TGV/PV) and compared with changes in barostat volume (fundal accommodation).
Results
Patients with functional dyspepsia showed a smaller change in proximal gastric volume than healthy subjects using barostat (mean (SD) 82 (61) v 205 (79) ml, p<0.01) and 3DUS (118 (41) v 199 (39) ml, p<0.01). Dysaccommodation of the proximal stomach was observed in 7 of 15 (46%) patients using the barostat technique. 10 of 15 (67%) patients were found to have an abnormal change in proximal gastric volume using TGV/PV ratio. At 5 min postprandially, fullness was related to the change in distal gastric volume (r = 0.51, p = 0.006) in the 3DUS study, whereas no relationship was observed in the barostat study.
Conclusion
3DUS is a feasible non‐invasive technique to measure gastric volumes and shows a distinct overlap with barostat data in healthy subjects and patients with functional dyspepsia. Fullness relates to distal gastric volumes when assessed by non‐invasive 3DUS.
Since the development of the barostat by Azpiroz,1,2,3 a large number of studies have investigated the role of motor, especially gastric tone, and sensory function of the proximal stomach in health and disease. In the physiological state, gastric accommodation can be described as the reduction in gastric tone and increase in compliance in response to meal ingestion.4,5 This physiological response facilitates ingestion of solids or liquids without considerable rise in intragastric pressure. An altered meal distribution was described within a subgroup of patients with functional dyspepsia.6 After this publication, several studies showed that impaired gastric accommodation can be observed in a subgroup of patients with functional dyspepsia,6,7,8,9,10,11 rumination syndrome12 and diabetes mellitus,13,14,15 and after gastric surgery.10 This impaired accommodation response2,16 is associated with upper gastrointestinal sensations such as early satiety and weight loss.17 Most studies investigating gastric accommodation applied the barostat technique, using an intragastric bag placed in the proximal stomach, to quantify gastric accommodation after meal ingestion. Using this technique, it has been shown that about 40% of the patients with functional dyspepsia have an impaired accommodation response.7 The barostat technique, however, has several disadvantages, such as invasiveness and effect on gastric physiology.18 These observations prompted the development of less invasive techniques to assess change in gastric volume as a reflection of gastric accommodation. These techniques include magnetic resonance imaging,19,20 single‐photon‐emission computed tomography,21,22,23,24,25 and two‐dimensional and three‐dimensional ultrasonography (3DUS).16,18,26,27,28,29,30,31,32 Recently 3DUS was introduced to assess gastric accommodation. Previous studies showed that 3DUS has a high accuracy in estimation of gastric volume33 and that gastric air pockets up to 20% of the total gastric volume do not interfere with 3DUS volumetry.16 In a subsequent study, we showed the reproducibility of three‐dimensional gastric volume estimations.34 However, to date no study has compared the results obtained by the barostat technique with those obtained by 3DUS in patients with functional upper gastrointestinal disorders.
The aim of this study was therefore to perform a head‐to‐head comparison of gastric postprandial volumes measured with the barostat technique and with 3DUS and to investigate the relationship between gastric volumes and upper gastrointestinal sensations in patients with functional dyspepsia and in healthy subjects.
Materials and methods
Study subjects
A total of 15 healthy subjects (6 men, age range 22–42 years, median 28 years) without any gastrointestinal symptoms or previous gastrointestinal surgery or disease and 15 patients with functional dyspepsia (5 men, age range 21–69 years, median 38 years; p = 0.06) were included in the study after obtaining written informed consent. The patients were selected on the basis of the Rome II criteria for functional dyspepsia. Organic disease was ruled out by means of upper gastrointestinal endoscopy, upper abdominal ultrasonography and routine biochemistry. The protocol was approved by the ethics committee of the University Medical Center Utrecht (Utrecht, The Netherlands). All participants were studied on two separate days in random order. On one study day, proximal gastric accommodation was studied using the barostat. On the other study day, proximal and distal gastric volume changes were studied using 3DUS. Studies were carried out at 09:00 h after fasting overnight.
Barostat technique
Proximal gastric volume changes were measured using the barostat technique. The participants were asked to sit on a bed, leaning slightly backward (at an angle of 110°). A polyethylene bag with a maximum capacity of 1000 ml attached to a double‐lumen tube (ventrol levin tube, outer diameter 6 mm, length 120 cm; Nellcor, California, USA) was introduced into the proximal stomach transorally and slowly unfolded by manual inflation of 200 ml of air.
The barostat bag was positioned in the proximal stomach by gently withdrawing the tube until resistance was felt.8,17,35,36,37 Thereafter, the bag was completely deflated and connected to the barostat device. After a 15‐min rest period, minimal distension pressure (MDP) was determined by increasing the pressure in the bag in 1‐mm Hg increments. MDP was defined as the lowest pressure level that provided a mean intrabag volume of 30 ml. For the remainder of the study, intrabag pressure was set at 2 mm Hg above MDP (MDP+2). A 15‐min equilibration period was followed by a baseline recording of 15 min. After this baseline period, participants were asked to drink 200 ml of Nutridrink (300 kcal, Nutricia, Zoetermeer, The Netherlands), consisting of 12 g protein, 11.6 g fat and 36.8 g carbohydrates, within 3 min using a straw. Barostatic bag volumes were monitored at MDP+2 for 60 min after the meal ingestion. Volume and pressure recordings were stored in a personal computer (sample frequency 1 Hz). After the study period, the barostat bag was deflated and removed. Before and during the barostat study (at 5, 15, 30, 45 and 60 min postprandially), sensations (hunger, nausea, fullness and epigastric pain) were scored on a 100‐mm‐long visual analogue scale on which 0 mm was no symptoms and 100 mm was unbearable symptoms. Proximal gastric dysaccommodation was defined as a fundal accommodation response (fundal accommodation = mean accommodation volume – mean baseline volume) less than −2 standard deviations (SD) of the mean fundal accommodation response observed in healthy subjects.7
3DUS technique
Participants were seated in a comfortable chair leaning slightly backward (at an angle of 110°). A 3DUS measurement of the total stomach was taken as described below. After this fasting ultrasound scan, a 500‐ml, 300‐kcal liquid meal (200 ml Nutridrink (1.5 kcal/ml); Nutricia, The Netherlands) mixed with 300 ml of water) was ingested within 3 min. At 5, 15, 30, 45 and 60 min postprandially, 3DUS measurements were repeated and sensations (hunger, nausea, fullness and epigastric pain) were scored using a visual analogue scale (as in the barostat technique).
A standard ultrasound scanner (Esaote‐Pie Medical, Maastricht, The Netherlands) with a 3.5‐MHz curved probe (Esaote‐Pie Medical, Maastricht, The Netherlands) with an attached position sensor (MedCom GmbH, Darmstadt, Germany) was used to perform this study. The probe was placed on the abdominal wall and the stomach was localised. The whole stomach was visualised using a fluent left‐to‐right lateral movement of the probe, from fundus to pylorus. During this sweep, about 400 sagittal images were digitised and stored on the personal computer using InVivo Scan NT software (MedCom GmbH, Darmstadt, Germany). During all sweeps, the position and the orientation of the ultrasound probe were recorded continuously by a magnetic tracker system. This system consists of a transmitter generating a pulse magnetic field and a receiver (sensor) attached to the ultrasound probe, defining the position and orientation in relation to the transmitter. The tracker transmitter was positioned at a maximum distance of 60 cm from the patient.
Using specialised software (InVivo), the computer calculated images of transverse and longitudinal planes on the basis of the information in the sagittal images and the measured position and orientation of the ultrasound probe.
Regions of interest (ROIs) were then drawn using the original sagittal ultrasound images. The ROIs were constructed using the inner layer of the gastric wall, measuring the gastric volume. After delineation of the gastric wall in the sagittal plane, the computer calculated ROIs in the other two planes and created a three‐dimensional image of the stomach (fig 1). The total gastric volume (TGV) was calculated using this three‐dimensional reconstruction. In addition, partial gastric volumes were calculated. The proximal gastric volume was calculated using a dividing plane 10 cm from the diaphragm downward, perpendicular to the longitudinal axis of the stomach. The gastric volume within this 10‐cm region represented the proximal part. The 10‐cm dimension was chosen on the basis of the mean maximum postprandial barostat bag volume being 500 ml. Using the formula volume = 4/3.π.r3, r was determined to be 4.9. The mean maximum diameter of the barostat bag was therefore 9.85, about 10 cm.
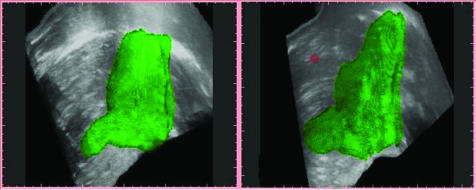
Figure 1 Three‐dimensional ultrasonographic images showing an example of the stomach of a healthy subject (left) and a patient with functional dyspepsia (right). Note the larger distal part and the smaller proximal part of the stomach in functional dyspepsia.
The distal antral volume was calculated by constructing a plane perpendicular to the antral axis at the point where the antrum, liver, superior mesenteric vein and the aorta could be seen simultaneously. The gastric volume from this plane to the pylorus represented the distal part. The volumes of the proximal and distal parts were calculated and used for further analysis. The third volume in our three‐compartment model, the corpus volume, was calculated by subtracting the distal volume and proximal volume from the total gastric volume. TGV, proximal volume, distal volume and corpus volume were compared between patients with functional dyspepsia and healthy subjects. To analyse dysaccommodation using our three‐dimensional data, total gastric volume/proximal volume (TGV/PV) ratios were calculated in all patients and compared with the upper normal range of the data of healthy subjects.
Statistical analysis
Data are presented as mean values (standard error of the mean), except when stated otherwise. Barostat and 3DUS variables were compared using a two‐sided Student's t test. The normal range (mean (2 SD)) for the meal‐induced gastric volume increase was calculated from the data obtained in healthy subjects. A multivariate analysis of variance (ANOVA) method was used to evaluate the effect of the repeated measurements of gastric volume. Relationships between gastrointestinal sensations and volume variables were assessed using Pearson's correlation coefficient. Significance was accepted at the 5% level. All statistical analyses were carried out with SPSS V.11.0 for MS Windows.
Results
Gastric accommodation using the barostat technique
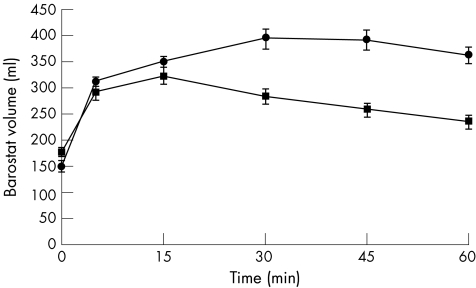
All healthy subjects and patients with functional dyspepsia completed the barostat study. MDP was comparable in both groups (healthy subjects mean (SD) 7.3 (0.42) mm Hg; patients with functional dyspepsia 7.2 (0.47) mm Hg; p = NS). In healthy subjects, the mean preprandial intragastric bag volume at MDP+2 was 150 (9.2) ml. In all healthy subjects, an immediate relaxation of the proximal stomach was seen after meal ingestion as shown by a barostat bag volume increase. At 5 min after the meal, the intrabag volume was significantly larger than the preprandial volume (150 (9.2) v 312 (16) ml; p<0.001). Mean fundal accommodation, calculated using the “Tack method”, in the healthy subjects was 215 (66) ml, with a lower range of normal of 83 ml.
In patients with functional dyspepsia, the mean (SD) preprandial intragastric bag volume (at MDP+2) was 195 (20) ml. At 5 min postprandially, a significant increase in volume was measured (195 (17) v 291 (21); p<0.001). The mean postprandial barostat bag volume in patients was significantly smaller than that in healthy subjects (ANOVA p = 0.001; fig 2). Mean increase in barostat volume was significantly smaller in patients with functional dyspepsia (82 (61) v 205 79 ml; p<0). On using the lower range of normal (83 ml) as a cut‐off point, 7 of 15 (47%) patients with functional dyspepsia showed an impaired proximal gastric accommodation response as measured by the barostat technique.
Figure 2 Mean intragastric bag volume in patients with functional dyspepsia (squares) and in healthy subjects (circles). Ingestion of the meal induces a rapid and sustained increase in intragastric bag volume in both functional dyspepsia and healthy subjects. However, in functional dyspepsia there is a significantly smaller bag volume than in healthy subjects (analysis of variance p = 0.001).
Gastric accommodation using 3DUS
The mean fasting total gastric volume was comparable in both groups (healthy subjects 32.5 (2.5) ml, patients with functional dyspepsia 44.4 (7.2) ml; p = NS). Mean fasting distal stomach volume was larger in patients with functional dyspepsia than in healthy subjects (5.6 (0.44) v 2.9 (0.26); p = 0.001).
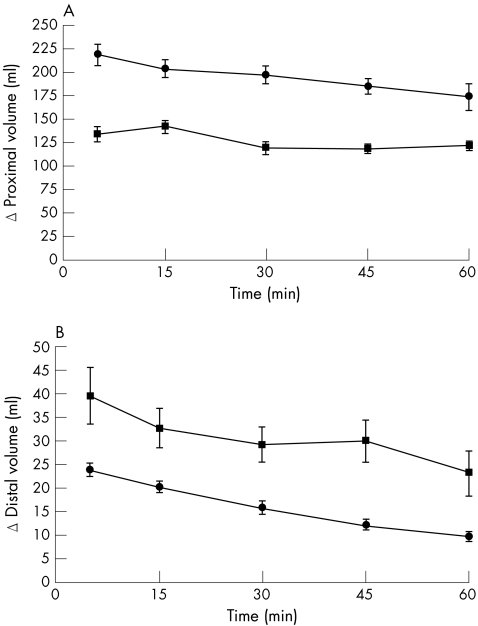
No difference was observed in TGV between both groups (ANOVA p = NS) in the first postprandial hour. During the first postprandial hour, proximal gastric volume was significantly smaller in patients with functional dyspepsia compared with healthy subjects (ANOVA p<0.001), although distal gastric volume was significantly larger in patients with functional dyspepsia than in healthy subjects (ANOVA p = 0.001; fig 3). The volume of the third compartment, the corpus volume, was significantly smaller in healthy subjects than in patients with functional dyspepsia (ANOVA p = 0.001) during the first postprandial hour. Given that TGV is comparable in both groups, this implies that the meal in patients with functional dyspepsia was shifted towards the corpus and distal stomach. The sum of distal volume and proximal volume was never larger than TGV; consequently, the distal and proximal gastric volumes did not overlap.
Figure 3 Mean proximal (A) and distal (B) volumes in patients with functional dyspepsia (squares) and in healthy subjects (circles). Note the significantly smaller proximal volume (ANOVA p<0.001) and significantly larger distal volume in patients with functional dyspepsia (ANOVA p = 0.001) compared with those in healthy subjects using 3D ultrasonography.
The mean (SD) TGV/PV ratio in healthy subjects was 2.12 (0.24). The cut‐off point was at 2.61. Thus, abnormal accommodation was observed in 10 of 15 (67%) patients. Six of these patients with abnormal accommodation were also detected by the barostat technique. The seventh patient detected as dysaccommodant by the barostat had a TGV/PV ratio of 2.60 and could be considered as borderline.
Relationship between three‐dimensional gastric volume and barostat volume data
TGV measured with 3DUS 5 min postprandially showed a significant, albeit weak, relation with barostat data (r = 0.37, p = 0.046). However, proximal volume showed a stronger correlation (r = 0.55, p = 0.002) with barostat data 5 min after the meal. Distal volume was not related to barostat volumes (r = −0.32, p = 0.08).
Upper gastrointestinal sensations
Barostat technique
Both in healthy subjects and in patients with functional dyspepsia, a significant increase in the sensation fullness (healthy subjects 3.5 (1.2) v 26.3 (5.6), p = 0.001; functional dyspepsia 7.9 (2.9) v 57.3 (6), p<0.001) and a decrease in the sensation hunger was observed (healthy subjects 30.5 (7.2) v 16.2 (5.1), p = 0.04; functional dyspepsia 35 (8.7) v 12 (5.9), p = 0.008) 5 min after the meal. The mean postprandial fullness and hunger were significantly different between healthy subjects and patients with functional dyspepsia (ANOVA p<0.001, ANOVA p = 0.03).
No significant changes occurred in nausea (healthy subjects 2.0 (0.78) v 5.0 (2.4), p = NS; patients with functional dyspepsia 11.4 (3.9) v 16.3 (6.4), p = NS) or upper abdominal pain (healthy subjects 0.5 (0.21) v 1.1 (0.4), p = NS; patients with functional dyspepsia 8.5 (3.3) v 21.3 (7.9), p = NS).
3DUS technique
In both patients and healthy subjects, an increase in the sensation fullness (healthy subjects 5.1 (2.4) v 61.3 (4.9), p<0.001; patients with functional dyspepsia 5.4 (2.0) v 65.5 (7.1), p <0.001) as well as a decrease in the sensation hunger (healthy subjects 37.4 (5.8) v 12 (3.9), p<0.001; patients with functional dyspepsia 45.2 (8.1) v 13.6 (5.6), p = 0.001) were observed 5 min after meal ingestion. The mean postprandial fullness and hunger were significantly different between healthy subjects and patients with functional dyspepsia (ANOVA p = 0.01, p = 0.05). No changes were observed in the sensations nausea (healthy subjects 5.9 (3.9) v 9.4 (3.9), p = NS; patients with functional dyspepsia 20.1 (6.8) v 25.7 (6.9), p = NS) and upper abdominal pain (healthy subjects 0.6 (0.25) v 0.7 (0.36), p = NS; patients with functional dyspepsia 21.6 (7.3) v 19 (7.1), p = NS).
Although both patients and healthy subjects showed a significant increase in fullness, in the 3DUS study, patients experienced fullness longer than healthy subjects. At 60 min, patients with functional dyspepsia still had a significantly higher fullness score than healthy subjects (37.9 (6.5) v 18.6 (2.9), p = 0.035).
Relationship between gastric volume data and gastrointestinal sensations
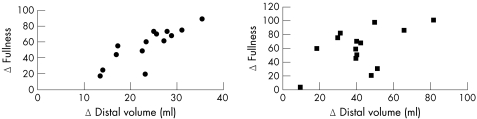
Barostat data showed a negative, non‐significant relation with the increase in fullness (r = −0.25, p = 0.18). Using the 3DUS technique, no relations were found between the increase in TGV and proximal volume and gastrointestinal sensations. When analysed separately, the increase in distal volume was related to fullness in healthy subjects (r = 0.78, p<0.001), whereas a borderline significant relation was seen in patients with functional dyspepsia (r = 0.52, p = 0.05) 5‐min postprandially (fig 4). No relationships were found with the sensations hunger and pain.
Figure 4 Scatterplot showing the relation between the increase in the sensation fullness and distal volume in healthy subjects (left; r = 0.78, p = 0.001) and patients with functional dyspepsia (right; r = 0.52, p = 0.05) 5 min postprandially.
Discussion
The major findings of the study are: (1) 3DUS can be used to identify patients with functional dyspepsia with impaired relaxation of the proximal stomach during a barostat experiment; (2) the change in distal gastric volume after ingestion of a nutrient meal is related to the sensation fullness.
In the present study, 47% of the patients with functional dyspepsia showed an impaired accommodation response to a liquid nutrient measured using the barostat. This is comparable with the 40% that was earlier described by Tack et al.7 The TGV/PV ratios calculated from 3DUS data showed impaired volume change of the proximal stomach in 10 of 15 (67%) patients. Six of 7 patients detected by the barostat as dysaccommodants were also picked up by 3DUS, whereas the seventh patient was borderline–normal. Thus, almost all patients with functional dyspepsia who were regarded as having dysaccommodation by the barostat study were identified using the non‐invasive ultrasonographic technique to assess changes in partial gastric volumes after ingestion of a liquid nutrient. In addition, 3DUS detected impaired proximal volume changes in some participants, with a normal accommodation response using the barostat. The normal accommodation response might be explained by the differences in the stimulus resulting in gastric accommodation. The barostat technique requires a bag positioned in the proximal stomach, which is kept at a constant pressure above the intra‐abdominal pressure, whereas the volume challenge in the two experiments is comparable; the pressure component is substantially different.18
This study showed that patients with functional dyspepsia have not only a smaller proximal gastric volume after meal ingestion but also a larger distal antral volume when assessed by 3DUS. Direct comparison of proximal volume assessed with 3DUS and barostat bag volume showed a correlation with an r value of 0.55, which indicates a clear relationship. However, it also indicates that the techniques are not interchangeable, which is likely because of the differences in invasiveness of both techniques.
Our finding that the distal gastric volume is larger in patients with functional dyspepsia is in line with the results of studies using 2DUS. Moreover, it confirms the observation by Caldarella et al38 of antrofundic dysfunctions in functional dyspepsia. Our primary aim was to compare the findings obtained from the barostat technique with the non‐invasive 3DUS technique. We therefore used a three‐compartment model in this study. Calculating a proximal volume compartment, which was comparable to the barostat bag volume, enabled us to compare both techniques optimally. By contrast, others used a two‐compartment model in which proximal and distal parts are calculated using a division line from the incisura angularis towards the greater curvature.33 A potential disadvantage of this method is that the incisura is not always visible and the choice of angle of the division line towards the greater curvature might influence the data. Future studies using 3DUS might give new information about the most ideal model of division of the different anatomical and physiological gastric parts, which will depict the differences between patients and healthy subjects most optimally.
An interesting finding in our study is that the change in distal antral volume is related to the sensation fullness. This relationship was stronger in healthy subjects than in patients with functional dyspepsia. This suggests that in health the interplay between distal gastric volume, tone and compliance plays an important part in the intensity of the sensation fullness, whereas generation of fullness in functional dyspepsia is a more complex phenomenon that is also modulated by other regions of the stomach or influenced by gastric sensitivity.39
In our study, the median age difference between healthy subjects and patients was, although not significantly different, 10 years. In a recent study by Delgado‐Aros et al,40 no effect of age was seen on gastric preprandial and postprandial volumes. In addition, in a 3DUS study conducted in our motility lab in which more that 100 subjects participated, no effect of age could be detected.47 Hence, the results of our study were probably not influenced by the difference in age.
Ultrasound imaging is used widely in clinical medicine. Its benefits include speed, low cost and the limited exposure risk. Although imaging in 3D is starting to become more common place in several medical fields—for example, cardiology41 and obstetrics42,43—most clinical scanning remains in 2D. In the gastroenterology field, 3DUS is a relatively new technique. The Bergen group showed that 3DUS enhances accuracy in volume estimation31 compared with 2DUS.44,45 In addition, using 3DUS, total stomach volume as well as partial gastric volumes can be calculated.
However, there are some potential disadvantages using 3DUS,46 such as air pockets that reside in the stomach. Tefera et al33 dealt with this point by classifying the amounts of air (0: no visible air, 1: small amounts, 2: moderate amounts and 3: large amounts, affecting the quality of ultrasonography and hence necessitating exclusion from the study). They showed in a study with 40 participants that none of the participants had to be excluded because of large air pockets, and that the presence of air did not influence the results. Recently, we showed that amounts of air up to 20% of the total gastric volume do not interfere with 3DUS volumetry,16 which confirms the findings of Gilja et al.28,29 The present study, which was carried out with participants in the seated position, disturbing air–fluid interfaces were not encountered.
The findings of our present study encourage the use of 3DUS in large groups of patients to investigate the characteristics of the human stomach in upper gastrointestinal diseases and to evaluate pharmacotherapy. These studies will increase our understanding of the pathophysiology of functional gastric disorders, especially functional dyspepsia and its relationship with upper gastrointestinal sensations.
In conclusion, 3DUS is a reliable non‐invasive technique of measuring gastric volumes and shows a large overlap with the findings in the evaluation of gastric physiology using the barostat in patients with functional dyspepsia. However, there are differences that need to be studied in terms of pathophysiological value and predictors of outcome during pharmacological interventions. Future studies using this technique may give important clinical information about the physiology and pathophysiology of functional gastric sensations. The upper gastrointestinal sensation fullness is related to distal gastric volume, but more factors might play a part in the generation of this sensation in functional dyspepsia.
Abbreviations
ANOVA - analysis of variance
3DUS - three‐dimensional ultrasonography
MDP - minimal distension pressure
ROIs - regions of interest
TGV/PV - total gastric volume/proximal volume ratio
Footnotes
Competing interests: None.
References
- 1.Azpiroz F, Malagelada J R. Isobaric intestinal distension in humans: sensorial relay and reflex gastric relaxation. Am J Physiol 1990258(Pt 1)G202–G207. [DOI] [PubMed] [Google Scholar]
- 2.Azpiroz F, Malagelada J R. Gastric tone measured by an electronic barostat in health and postsurgical gastroparesis. Gastroenterology 198792934–943. [DOI] [PubMed] [Google Scholar]
- 3.Azpiroz F, Malagelada J R. Importance of vagal input in maintaining gastric tone in the dog. J Physiol 1987384511–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azpiroz F, Malagelada J R. Intestinal control of gastric tone. Am J Physiol 1985249(Pt 1)G501–G509. [DOI] [PubMed] [Google Scholar]
- 5.Azpiroz F. Gastric tone and the barostat: comprehend and compromise. Neurogastroenterol Motil 199795. [DOI] [PubMed] [Google Scholar]
- 6.Troncon L E, Bennett R J, Ahluwalia N K.et al Abnormal intragastric distribution of food during gastric emptying in functional dyspepsia patients. Gut 199435327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tack J, Piessevaux H, Coulie B.et al Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology 19981151346–1352. [DOI] [PubMed] [Google Scholar]
- 8.Salet G A, Samsom M, Roelofs J M.et al Responses to gastric distension in functional dyspepsia. Gut 199842823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thumshirn M, Camilleri M, Saslow S B.et al Gastric accommodation in non‐ulcer dyspepsia and the roles of Helicobacter pylori infection and vagal function. Gut 19994455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wijnhoven B P, Salet G A, Roelofs J M.et al Function of the proximal stomach after Nissen fundoplication. Br J Surg 199885267–271. [DOI] [PubMed] [Google Scholar]
- 11.Hausken T, Svebak S, Wilhelmsen I.et al Low vagal tone and antral dysmotility in patients with functional dyspepsia. Psychosom Med 19935512–22. [DOI] [PubMed] [Google Scholar]
- 12.Thumshirn M, Camilleri M, Hanson R B.et al Gastric mechanosensory and lower esophageal sphincter function in rumination syndrome. Am J Physiol 1998275(Pt 1)G314–G321. [DOI] [PubMed] [Google Scholar]
- 13.Samsom M, Salet G A, Roelofs J M.et al Compliance of the proximal stomach and dyspeptic symptoms in patients with type I diabetes mellitus. Dig Dis Sci 1995402037–2042. [DOI] [PubMed] [Google Scholar]
- 14.Samsom M, Jebbink H J, Smout A J.et al Disorders in the motility of stomach and small intestine in patients with insulin‐dependent diabetes. Ned Tijdschr Geneeskd 19931372068–2072. [PubMed] [Google Scholar]
- 15.Undeland K A, Hausken T, Gilja O H.et al Gastric meal accommodation studied by ultrasound in diabetes. Relation to vagal tone. Scand J Gastroenterol 199833236–241. [DOI] [PubMed] [Google Scholar]
- 16.Scheffer R C, Gooszen H G, Wassenaar E B.et al Relationship between partial gastric volumes and dyspeptic symptoms in fundoplication patients: a 3D ultrasonographic study. Am J Gastroenterol 2004991902–1909. [DOI] [PubMed] [Google Scholar]
- 17.Tack J, Caenepeel P, Fischler B.et al Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology 2001121526–535. [DOI] [PubMed] [Google Scholar]
- 18.Mundt M W, Hausken T, Samsom M. Effect of intragastric barostat bag on proximal and distal gastric accommodation in response to liquid meal. Am J Physiol Gastrointest Liver Physiol 2002283G681–G686. [DOI] [PubMed] [Google Scholar]
- 19.Schwizer W, Maecke H, Fried M. Measurement of gastric emptying by magnetic resonance imaging in humans. Gastroenterology 1992103369–376. [DOI] [PubMed] [Google Scholar]
- 20.Marciani L, Gowland P A, Spiller R C.et al Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI. Am J Physiol Gastrointest Liver Physiol 2001280G1227–G1233. [DOI] [PubMed] [Google Scholar]
- 21.Bouras E P, Delgado‐Aros S, Camilleri M.et al SPECT imaging of the stomach: comparison with barostat, and effects of sex, age, body mass index, and fundoplication. Single photon emission computed tomography. Gut 200251781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuiken S D, Samsom M, Camilleri M.et al Development of a test to measure gastric accommodation in humans. Am J Physiol 1999277(Pt 1)G1217–G1221. [DOI] [PubMed] [Google Scholar]
- 23.Liau S S, Camilleri M, Kim D Y.et al Pharmacological modulation of human gastric volumes demonstrated noninvasively using SPECT imaging. Neurogastroenterol Motil 200113533–542. [DOI] [PubMed] [Google Scholar]
- 24.Kim D Y, Camilleri M. Noninvasive measurement of gastric accommodation by SPECT. Korean J Intern Med 2002171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Elzen B D, Bennink R J, Wieringa R E.et al Fundic accommodation assessed by SPECT scanning: comparison with the gastric barostat. Gut 2003521548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones K L, Doran S M, Hveem K.et al Relation between postprandial satiation and antral area in normal subjects. Am J Clin Nutr 199766127–132. [DOI] [PubMed] [Google Scholar]
- 27.Hausken T, Berstad A. Wide gastric antrum in patients with non‐ulcer dyspepsia. Effect of cisapride. Scand J Gastroenterol 199227427–432. [DOI] [PubMed] [Google Scholar]
- 28.Gilja O H, Hausken T, Odegaard S.et al Monitoring postprandial size of the proximal stomach by ultrasonography. J Ultrasound Med 19951481–89. [DOI] [PubMed] [Google Scholar]
- 29.Gilja O H, Hausken T, Wilhelmsen I.et al Impaired accommodation of proximal stomach to a meal in functional dyspepsia. Dig Dis Sci 199641689–696. [DOI] [PubMed] [Google Scholar]
- 30.Hausken T, Sondenaa K, Svebak S.et al Common pathogenetic mechanisms in symptomatic, uncomplicated gallstone disease and functional dyspepsia: volume measurement of gallbladder and antrum using three‐dimensional ultrasonography. Dig Dis Sci 1997422505–2512. [DOI] [PubMed] [Google Scholar]
- 31.Gilja O H, Hausken T, Odegaard S.et al Three‐dimensional ultrasonography of the gastric antrum in patients with functional dyspepsia. Scand J Gastroenterol 199631847–855. [DOI] [PubMed] [Google Scholar]
- 32.Gilja O H, Detmer P R, Jong J M.et al Intragastric distribution and gastric emptying assessed by three‐dimensional ultrasonography. Gastroenterology 199711338–49. [DOI] [PubMed] [Google Scholar]
- 33.Tefera S, Gilja O H, Olafsdottir E.et al Intragastric maldistribution of a liquid meal in patients with reflux oesophagitis assessed by three dimensional ultrasonography. Gut 200250153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mundt M W, Hausken T, Smout A J P M.et al Gastric volume measured by 3D ultrasonography; the effect of glucagon on gastric volumee. Gastroenterology 2001120A247 [Google Scholar]
- 35.Samsom M, Roelofs J M, Akkermans L M.et al Proximal gastric motor activity in response to a liquid meal in type I diabetes mellitus with autonomic neuropathy. Dig Dis Sci 199843491–496. [DOI] [PubMed] [Google Scholar]
- 36.Boeckxstaens G E, Hirsch D P, Kuiken S D.et al The proximal stomach and postprandial symptoms in functional dyspeptics. Am J Gastroenterol 20029740–48. [DOI] [PubMed] [Google Scholar]
- 37.Boeckxstaens G E, Hirsch D P, van den Elzen B D.et al Impaired drinking capacity in patients with functional dyspepsia: relationship with proximal stomach function. Gastroenterology 20011211054–1063. [DOI] [PubMed] [Google Scholar]
- 38.Caldarella M P, Azpiroz F, Malagelada J R. Antro‐fundic dysfunctions in functional dyspepsia. Gastroenterology 20031241220–1229. [DOI] [PubMed] [Google Scholar]
- 39.Mearin F, Cucala M, Azpiroz F.et al The origin of symptoms on the brain‐gut axis in functional dyspepsia. Gastroenterology 1991101999–1006. [DOI] [PubMed] [Google Scholar]
- 40.Delgado‐Aros S, Cremonini F, Castillo J E.et al Independent influences of body mass and gastric volumes on satiation in humans. Gastroenterology 2004126432–440. [DOI] [PubMed] [Google Scholar]
- 41.Naqvi T Z. Recent advances in echocardiography. Expert Rev Cardiovasc Ther 2004289–96. [DOI] [PubMed] [Google Scholar]
- 42.Baba K. Obstetrics and gynecology. Nippon Rinsho 200462807–814. [PubMed] [Google Scholar]
- 43.Timor‐Tritsch I E, Platt L D. Three‐dimensional ultrasound experience in obstetrics. Curr Opin Obstet Gynecol 200214569–575. [DOI] [PubMed] [Google Scholar]
- 44.Gilja O H, Smievoll A I, Thune N.et al In vivo comparison of 3D ultrasonography and magnetic resonance imaging in volume estimation of human kidneys. Ultrasound Med Biol 19952125–32. [DOI] [PubMed] [Google Scholar]
- 45.Gilja O H, Thune N, Matre K.et al In vitro evaluation of three‐dimensional ultrasonography in volume estimation of abdominal organs. Ultrasound Med Biol 199420157–165. [DOI] [PubMed] [Google Scholar]
- 46.Schwizer W, Steingotter A, Fox M.et al Non‐invasive measurement of gastric accommodation in humans. Gut 200251(Suppl 1)I59–I62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lelyveld Nv, Scheffer B, Mundt M W.et al Impaired proximal gastric volume change in patients with functional dyspepsia is related to fullness; a 3d‐ultrasonography study. Abstract. DDW, USA: Chigaco, IL, 2005