Abstract
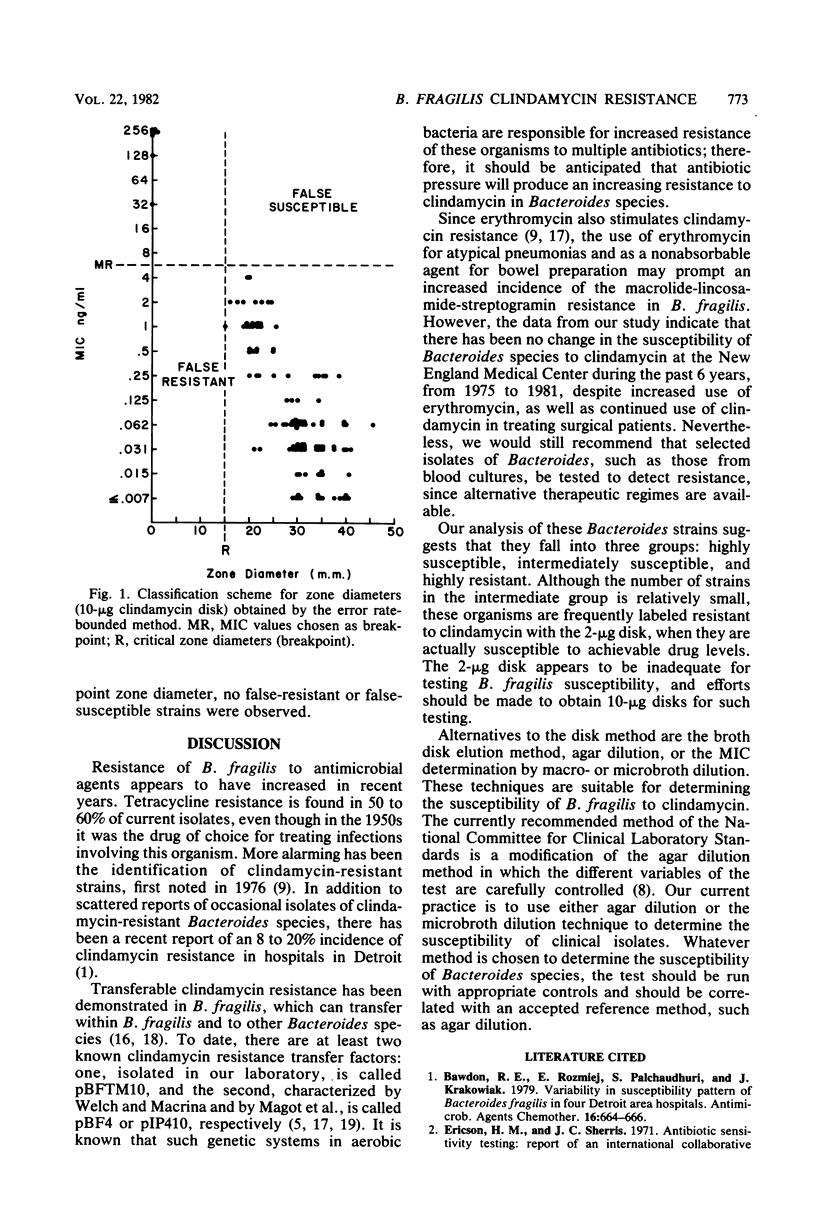
Clindamycin resistance in Bacteroides fragilis was examined in 507 strains isolated from 1973 to 1981. Three groups were recognized: highly susceptible (minimum inhibitory concentration [MIC] less than or equal to 0.125 microgram/ml), intermediately susceptible (MIC = 0.25 to 4 micrograms/ml), and highly resistant to (MIC greater than or equal to 8 microgram/ml). The incidence of high-level resistance (1.8%) had not changed during this period. Only 8 of 17 isolates reputed to be highly clindamycin resistant that were referred to our laboratory proved to be highly resistant (MICs greater than or equal to 32 microgram/ml), whereas the other 9 were intermediately susceptible. Analysis of 2- and 10-microgram clindamycin disks for determining the susceptibility of B. fragilis revealed a high false-resistance rate with the 2-microgram disk, most errors occurring with the intermediate group. There was no false resistance with the 10-microgram disk. When disk diffusion susceptibility of B. fragilis is employed, we recommend the 10-microgram disk to predict accurately the susceptibility of B. fragilis to clindamycin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bawdon R. E., Rozmiej E., Palchaudhuri S., Krakowiak J. Variability in the susceptibility pattern of Bacteroides fragilis in four Detroit area hospitals. Antimicrob Agents Chemother. 1979 Nov;16(5):664–666. doi: 10.1128/aac.16.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach S. L., Bartlett J. G. Anaerobic infections. 1. N Engl J Med. 1974 May 23;290(21):1177–1184. doi: 10.1056/NEJM197405232902106. [DOI] [PubMed] [Google Scholar]
- Magot M., Fayolle F., Privitera G., Sebald M. Transposon-like structures in the Bacteroides fragilis MLS plasmid plP 410. Mol Gen Genet. 1981;181(4):559–561. doi: 10.1007/BF00428754. [DOI] [PubMed] [Google Scholar]
- Martin W. J., Gardner M., Washington J. A., 2nd In vitro antimicrobial susceptibility of anaerobic bacteria isolated from clinical specimens. Antimicrob Agents Chemother. 1972 Feb;1(2):148–158. doi: 10.1128/aac.1.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler C. M., DeHaan R. M. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J Infect Dis. 1974 Dec;130(6):588–594. doi: 10.1093/infdis/130.6.588. [DOI] [PubMed] [Google Scholar]
- Privitera G., Dublanchet A., Sebald M. Transfer of multiple antibiotic resistance between subspecies of Bacteroides fragilis. J Infect Dis. 1979 Jan;139(1):97–101. doi: 10.1093/infdis/139.1.97. [DOI] [PubMed] [Google Scholar]
- Sutter V. L., Barry A. L., Wilkins T. D., Zabransky R. J. Collaborative evaluation of a proposed reference dilution method of susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1979 Oct;16(4):495–502. doi: 10.1128/aac.16.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Kwok Y. Y., Finegold S. M. Standardized antimicrobial disc susceptibility testing of anaerobic bacteria. I. Susceptibility of Bacteroides fragilis to tetracycline. Appl Microbiol. 1972 Feb;23(2):268–275. doi: 10.1128/am.23.2.268-275.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Jacobus N. V., Bartlett J. G., Gorbach S. L. Susceptibility of anaerobes to cefoxitin and other cephalosporins. Antimicrob Agents Chemother. 1975 Feb;7(2):128–132. doi: 10.1128/aac.7.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Snydman D. R., Gorbach S. L., Malamy M. H. Plasmid-mediated, transferable resistance to clindamycin and erythromycin in Bacteroides fragilis. J Infect Dis. 1979 Jan;139(1):83–88. doi: 10.1093/infdis/139.1.83. [DOI] [PubMed] [Google Scholar]
- Tally F. P., Snydman D. R., Shimell M. J., Malamy M. H. Characterization of pBFTM10, a clindamycin-erythromycin resistance transfer factor from Bacteroides fragilis. J Bacteriol. 1982 Aug;151(2):686–691. doi: 10.1128/jb.151.2.686-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Jones K. R., Macrina F. L. Transferable lincosamide-macrolide resistance in Bacteroides. Plasmid. 1979 Apr;2(2):261–268. doi: 10.1016/0147-619x(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Macrina F. L. Physical characterization of Bacteroides fragilis R plasmid pBF4. J Bacteriol. 1981 Feb;145(2):867–872. doi: 10.1128/jb.145.2.867-872.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


