Abstract
Background
Activation of the nuclear factor κB (NF‐κB) system is a major event in acute and chronic inflammatory processes. NF‐κB cascades are comprised of IκB kinases, IκBs and NF‐κB dimers. Little is known of the individual roles of these proteins in organ specific inflammation. The aim of the present study was to analyse the consequences of ectopic IκB kinase‐2 (IKK2) activation in the pancreas of mice.
Methods
Transgenic mice were generated using an inducible genetic system (tet system) to conditionally overexpress a gain of function mutant of IKK2 (tetO‐IKK2‐EE) in the pancreas. To achieve transgene expression in the pancreas, these animals were crossed with CMV‐rtTA mice that are known to express the rtTA protein in the pancreas.
Results
In these double transgenic animals, doxycycline treatment induced expression of IKK2‐EE (IKK2CA) in pancreatic acinar cells resulting in moderate activation of the IκB kinase complex, as measured by the immune complex kinase assay, and up to 200‐fold activation of the transgene expression cassette, as detected by luciferase assay. IKK2CA expression in the pancreas had a mosaic appearance. Ectopic IKK2CA mostly activated the classical NF‐κB pathway. The activation level of the NF‐κB cascade induced by IKK2CA was considerably lower compared with that observed after supramaximal caerulein stimulation but still led to the formation of leucocyte infiltrates first observed after 4 weeks of doxycycline stimulation with a maximum after 8–12 weeks. The infiltrates were mainly composed of B lymphocytes and macrophages. Increased mRNA levels of tumour necrosis factor α and RANTES were detected in pancreatic acinar cells. However, only minor damage to pancreatic tissue was observed. A combination of supramaximal caerulein stimulation with induction of IKK2CA caused increased tissue damage compared with either IKK2CA or caerulein alone.
Conclusions
Our observations suggest that the role of IKK2 activation in pancreatic acini is to induce leucocyte infiltration, but at a moderate level of activation it is not sufficient to induce pancreatic damage in mice. The IKK2CA induced infiltrations resemble those observed in autoimmune pancreatitis, indicating a role for IKK2/NF‐κB in this disease. IKK2CA in pancreatic acinar cells increases tissue damage of secretagogue induced experimental pancreatitis underlining the proinflammatory role of the IKK/NF‐κB pathway in this disease.
Activation of the transcription factor nuclear factor κB (NFκB) is implicated in a wide variety of inflammatory processes and malignancies.1 Several cascades mediate activation of NF‐κB in the nucleus. Most pathways converge at the IκB kinase (IKK).2 This multiprotein complex consists of the catalytic components IKK1 (α), heterodimerising with IKK2 (β) and the regulatory factors IKKγ/NEMO and ELKS.3,4,5 The main function of the IKK complex is to phosphorylate IκB proteins, which retain members of NF‐κB in an inactive cytosolic state. The NF‐κB family consists of the RelA, RelB, cRel, NF‐κB1 and NF‐κB2 proteins which can form homo‐ and heterodimers. On de novo phosphorylation of specific serines, IκB molecules are degraded and NF‐κB dimers are liberated, enter the nucleus and induce gene transcription. This multistep process is strongly regulated at all levels. Phosphorylation of IκB is one of the crucial regulatory elements in the “classical” NF‐κB cascade, which is activated in response to tumour necrosis factor α (TNFα) or interleukin‐1.6,7 Apart from this canonical pathway, alternative NF‐κB activation leads to phosphorylation of p100 and subsequent activation of RelB containing dimers. This alternative pathway mainly involves IKK1, whereas IKK2 and NEMO are crucial for the classical NF‐κB cascade.8,9
Activation of NF‐κB is an early event in experimental models of acute pancreatitis. Supramaximal doses of caerulein activate NF‐κB in pancreatic acinar cells both in vivo and in vitro. Several studies provide evidence for involvement of protein kinase C, Ras and the activation of trypsin in this process.10,11,12,13 We and others showed that the activity of IKK2 is transiently increased in response to supramaximal cholecystokinin.12,14 This observation still leaves the question of the role of NF‐κB in acute pancreatitis. Several studies using inhibitors or transduction with active components of NF‐κB into the pancreas suggest that activation of the NF‐κB signalling can have deleterious effects in experimental pancreatitis.15 Other reports indicate protective consequences as pharmacological inhibition of NF‐κB worsened secretagogue induced pancreatitis in vivo.10 Activation of the NF‐κB cascade has also been implicated in autoimmune diseases of the gastrointestinal tract. In inflammatory bowel disease, activation of the canonical pathway by TNFα is important for disease propagation and represents a therapeutic target.16,17 In gut endothelial cells, NF‐κB can protect from radiation induced cell injury.18 The role of NF‐κB in chronic inflammatory states of the pancreas such as alcoholic or autoimmune pancreatitis is unclear. Involvement of the NF‐κB system is likely as expression of NF‐κB target genes is reported in chronic alcoholic pancreatitis.19,20 The question of how individual components of the NF‐κB system participate in disease mechanisms remains largely unanswered. It is not clear whether IKK activation is important for the pathological alterations found in experimental pancreatitis. Therefore, the aim of the present study was to determine the role of active IKK2 in pancreatic cells in vivo.
Experimental procedures
Construction of the DNA transgene and generation of transgenic animals
The IKK2EE (S177E, S181E) coding sequence (kindly provided by Mercurio et al4) was cloned into the tet‐on‐vector pBI5.21,22,23 For the generation of transgenic mice, the bidirectional minigene was released by AatII‐AseI from pBI5‐IKK2EE. After purification, DNA was microinjected into pronuclei of fertilised oocytes (C57BL6/DBA) which were transplanted into pseudopregnant foster mothers (NMRI outbreed). Founder mice were identified by polymerase chain reaction (PCR) of genomic mouse tail DNA using the primers 5′‐GGT ACC CGG GGA TCC TCT AGT CAG‐3′ and 5′‐GGT CAC TGT GTA CTT CTG CTG CTC CAG‐3′ amplifying a 672 bp fragment specific for the IKK2EE (IKK2CA) transgene. More than 10 founder lines were obtained but only the two lines which showed the strongest transgene expression when the bidirectional promoter was activated by tTA from the line α‐CamKII‐tTA24,25 were included in this study (luciferase‐tetO7‐IKK‐2‐EE‐5 and EE‐7). To achieve IKK2EE expression in the pancreas, EE‐5 and EE‐7 mice were crossed with the line rtTACMV‐3.26 Animals transgenic for both transgenes (rtTA and luciferase‐tetO7‐IKKEE) were exposed to doxycycline by adding the drug to the drinking water (2 mg/ml 1% sucrose, light protected). Secretagogue induced pancreatitis was achieved by hourly intraperitoneal injection of caerulein at a total concentration of 100 μg/kg body weight. Mice were sacrificed 6–18 h after the first caerulein injection.
Determination of transgene expression
Cellular extracts were prepared from snap frozen tissue samples, homogenised in 500 μl of ice cold buffer (Dignam C27) using an ultraturrax and cleared by centrifugation (15 min, 15000 g, 4°C). The supernatant was assayed for luciferase activity and adjusted for protein content (light units per μg of total protein), as described previously.27 The western blot procedure was performed essentially as described previously.16,28
Immune complex kinase assays
IKK kinase assays were performed as described previously with minor modifications.27,28 Immunoprecipitation was performed from cellular lysates (0.5–1 mg of protein) using 2 μg of anti‐IKKα/β antibody (sc‐7607; Santa Cruz Biotechnology, Santa Cruz, California, USA). The reaction was performed in kinase buffer containing 500 ng of recombinant GST‐IκB as substrate16 in the presence of 10 μCi (0.185 MBq) [32P]ATP for 30 min at room temperature. The reaction was stopped by adding boiling laemmli. Lysates for western blotting or immunoprecipitates were separated by sodium dodecyl sulphate‐polyacrylamide gel electrophoresis. Activity was detected using Hyperfilm (Amersham Biosciences, Germany).
Preparations of tissue specimen, in situ histochemistry and immunofluorescence
Mice were killed by cervical dislocation. Blood samples were centrifuged for 10 min at 300 g. After bleeding, the tissue specimens were snap frozen or fixed in formalin and embedded in paraffin. Sections were stained with haematoxylin‐eosin and analysed by light microscopy. Evaluation of pancreatic sections was performed by three individual investigators. Sections were investigated for the presence of infiltrates, lymph node enlargement, oedema, vacuolisation and necrosis. Immunohistochemistry was performed as described previously.29,30
For immunohistochemistry the following antibodies were used: biotinylated α‐mouse or α‐rat IgG (Vector; Vector Labs, Burlingame, California, USA), CD‐3 and Ki 67 (Novocastra; Newcastle Upon Tyne, UK), CD 4, CD 45 and CD 8 (Pharmingen, Heidelberg, Germany), cytokeratin 19, p65 and p50 (Abcam, Cambridge, UK), IKK2 (Cell Signaling, Danvers, Massachusetts, USA), NCL‐MAC387 (Alexis) and luciferase (Novus‐Biologicals, Littleton, Colorado, USA).
Frozen sections were fixed in cold acetone, dried at room temperature, washed in phosphate buffered saline and blocked in 5% goat serum (in phosphate buffered saline) for 1 h. Sections were incubated with PE conjugated antibodies (B220 and CD11b; Becton Dickinson, Heidelberg, Germany) for 1 h (1: 200, diluted in blocking solution). Sections were washed three times in phosphate buffered saline and covered with mounting medium for AEC.
Isolation of RNA and laser capture microscopy
Snap frozen pancreatic tissue was pulverised in liquid nitrogen and total RNA was extracted using RNeasy Midi Kit (Qiagen, Hilden, Germany). Acinar cells were isolated from frozen sections by laser capture microscopy. RNA from isolated acinar sections was purified using the PicoRNA kit and further amplified using the RNA amplification kit (MessageAmp aRNA; Ambion, Darmstadt, Germany). Reverse transcription was performed using either 2 μg of total RNA or approximately 10 ng of amplified acinar mRNA with the Omniscript RT Kit (Qiagen) at 37°C for 1 h.
Real time reverse transcription‐polymerase chain reaction (RT‐PCR)
Real time PCR analysis was performed using either c‐DNA from total RNA (diluted 1:8) or c‐DNA from acinar RNA (non‐diluted): 20 μl of 2× Syber Green PCR Master Mix, 4 μl of cDNA and 300 nM primer mixture were topped up with water to a final volume of 40 μl. Amplification was performed under the following conditions: 2 min at 50°C, 10 min at 95°C, 15 s at 95°C and 1 min at 60°C for 45 cycles. The following primers were used: cyclophilin (F‐5′‐ATG GTC AAC CCC ACC GTG T‐3′, R‐5′‐TTC TGC TGT CTT TGG AAC TTT GTC‐3′); TNFα (F‐5′‐ATG AGA AGT TCC CAA ATG GCC‐3′, R‐5′‐TCC ACT TGG TGG TTT GCT ACG‐3′); MCP‐1 (F‐5′‐GGC TCA GCC AGA TGC AGT TAA‐3′, R‐5′‐CCT ACT CAT TGG GAT CAT CTT GCT3′); and RANTES (F‐5′‐GTG CTC CAA TCT TGC AGT CGT‐3′, R‐5′‐TGA ACC CAC TTC TTC TCT GGG T‐3′).
Results
Conditional expression of constitutive active IKK2 in the pancreas
To obtain an inducible model for IKK2CA expression in the pancreas, we crossed CMV‐tet‐R‐VP16 (rtTA) and tet‐(O)‐IKK2‐EE/luciferase mice. Figure 1 shows a scheme of the model system used in the study. As described in the experimental procedures section, the presence of doxycycline induces transcription of both luciferase and IKK2CA via binding of the transactivator to the bidirectional promoter. Transgenic mice were treated with doxycycline for a maximum of 3 months. Animals carrying both transgenes show virtually no luciferase activity in the absence of doxycycline. In addition, luciferase activity could not be detected in animals that were transgenic for luciferase or IKK2CA alone, either before or after administration of doxycycline. These latter types of animals were used as controls, as indicated.
Figure 1 Scheme of the inducible transgenic mouse model. Double transgenic mice CMV‐rtTA/Luciferase‐tet(O)‐IKK2‐EE were obtained by crossing CMV‐rtTA with luciferase‐tet(O)‐IKK2‐EE animals. Administration of doxycycline (Dox) induced conformational changes to rtTA enabling its ligation to the tet binding site to induce transcription of both IKK2‐EE (IKK2CA) and luciferase in double transgenic animals.
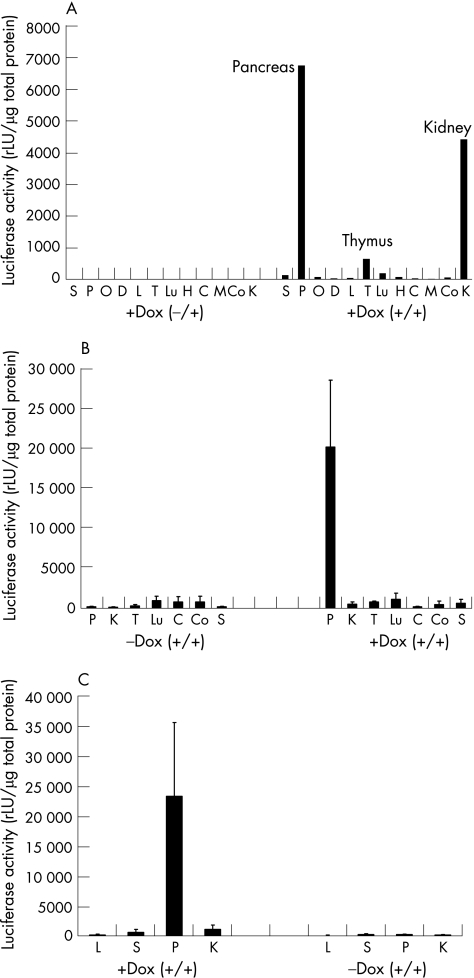
To test inducible organ specific transgene expression, luciferase activity was measured in various organs of double transgenic animals, including the pancreas, liver, spleen, lung and kidney. As shown in fig 2, luciferase activity was greatly increased in the pancreas of animals treated with doxycycline whereas the signals from the liver, spleen, lung, heart and brain regions remained low. However, we found slightly increased luciferase activity in the thymus and high activity in the kidney at the beginning of treatment (fig 2A). Nevertheless, when observed after 8–12 weeks of doxycycline treatment, luciferase activity in the double transgenic animals was detectable only in pancreatic lysates (fig 2B, C).
Figure 2 Tissue specific luciferase expression in CMV‐rtTA/Luciferase‐tet(O)‐IKK2‐EE mice. (A) Luciferase activity was measured in various organs of double transgenic mice (+/+) stimulated with doxycycline (+Dox) for 4 weeks. S, spleen; P, pancreas; O, ovar; D, duodenum; L, liver; T, thymus; Lu, lung; H, heart; C, cerebellum; M, midbrain; Co, cortex; K, kidney. Mean activity is presented as relative light units per μg of total protein (rLU/μg). Single transgenic mice (+/−) were used as controls (left panel). (B) Comparison of mean luciferase values in various organs after 8 weeks. (C) Mean luciferase counts after 12 weeks (n = 3 mice per group, values were measured in duplicate).
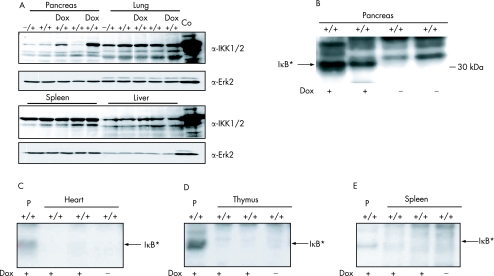
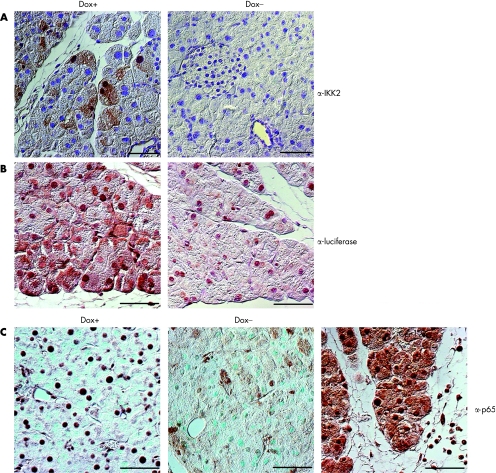
We analysed protein expression by western blot and found that the IKK2CA transgene was present only in the pancreas of double transgenic animals that had been treated with doxycycline for 8 weeks. No transgene expression was observed in the liver, spleen or lung of these treated animals, and single transgenic animals also showed no signs of increased IKK2 expression (fig 3A). Immune complex kinase assays revealed that the increase in IKK2 protein expression in the pancreas correlated with an increase in IKK activity. We used recombinant IκBα as a substrate in these assays and the specificity of the reaction was tested using a mutant form of the substrate where serines 32 and 36 were replaced by alanines. Increased IKK activity was not detectable in other organs such as the thymus, heart or spleen (fig 3B–E). To confirm these results, we performed immunohistochemistry to quantify protein expression of both transgenes IKK2 and luciferase in the pancreas of doxycycline stimulated animals. Figure 4A and 4B shows that IKK2 and luciferase is expressed mainly in pancreatic acinar cells. Interestingly, there is a mosaic expression pattern throughout the pancreas, indicating different protein levels of the transgenes in individual cells. To test whether IKK complex activation results in increased NF‐κB activity, we analysed tissue sections for the presence of nuclear p65, an indicator of canonical NF‐κB activity. Pancreatic sections of mice treated with supramaximal caerulein were used as a positive control. Figure 4C shows that doxycycline activation resulted in increased nuclear localisation of p65 in pancreatic acinar cells. Interestingly, the level of nuclear p65 in acinar cells was apparently lower in comparison with caerulein induced p65 expression (fig 4C). Taken together, these results demonstrate organ specific expression and function of constitutively active IKK2 in the pancreas of our double transgenic animals.
Figure 3 Expression and activity of IKK2‐EE (IKK2 CA) in CMV‐rtTA/luciferase‐tet(O)‐IKK2‐EE mice. (A) Protein expression of IKK 1/2 and Erk2 in pancreas, lung, spleen and liver of mice stimulated for 8 weeks with doxycycline (Dox) compared with unstimulated control mice. Recombinant IKK2 was used as a positive control (Co). +/− and +/+ represent single transgenic and double transgenic mice, respectively. (B) Activity of the immunopurified endogenous IKK complex in pancreatic lysates of non‐stimulated (Dox−) mice and mice stimulated for 8 weeks (Dox+), measured by in vitro phosphorylation of recombinant GST‐IκBα. The arrow indicates the phosphorylated (*) GST‐IκBα. (C) Immune complex kinase assay of the endogenous IKK complex purified from the heart after 8 weeks of doxycycline treatment, as described previously. IKK activity of the pancreatic lysate (P) of a mouse treated with doxycycline was used as a positive control. (D) Comparison of the IKK activity in the thymus after 8 weeks of doxycycline with the catalytic activity of IKK in the pancreas (P) (E) Immune complex kinase assay of IKK activity in the spleen after 8 weeks of doxycycline. Lysate from the pancreas (P) was used as a positive control.
Figure 4 Expression of the inducible IKK2‐EE/luciferase gene in pancreatic tissue. Ectopic expression of IKK2 CA (A) and luciferase (B) in mice treated with doxycycline (Dox +) compared with unstimulated control animals (Dox−). Expression of the transgene (IKK2 or luciferase) is indicated by brown precipitation in the tissue. Note that for both transgenes there is a mosaic expression pattern in pancreatic acinar cells. (C) Staining for the nuclear factor κB subunit p65 in pancreatic tissue in mice treated with doxycycline compared with unstimulated controls (Dox−) or mice treated with supramaximal caerulein to induce experimental pancreatitis. Note that there is moderate expression of nuclear (active) p65 in pancreatic acinar cells of doxycycline treated animals; expression level is considerable lower compared with caerulein treatment. One representative section of 3–4 mice analysed per group is displayed.
Cellular infiltrations in the pancreas are due to IKK2‐EE
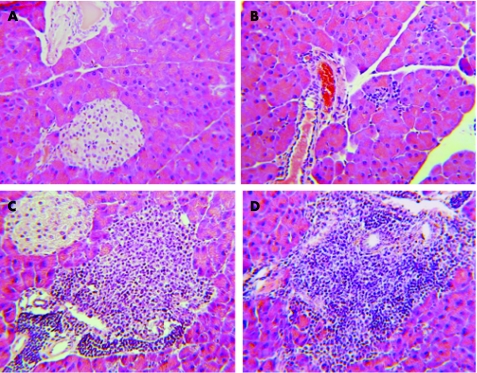
We next investigated the consequences of constitutive IKK activation in the pancreas. Histological analysis showed sparse infiltration of immune cells into the pancreatic tissue of double transgenic animals after 4 weeks of doxycycline treatment (fig 5). These immune cell infiltrates increased with time and formed confluent infiltrations, mainly around pancreatic ducts or vessels (fig 5). Islets of Langerhans were not surrounded by the infiltrating immune cells. No infiltrates were detected in untreated animals or in single transgenic mice treated with doxycycline. Further light microscopic analysis of the pancreas sections from double transgenic animals treated with doxycycline revealed no signs of destruction or necrosis of acinar cells or other cell types. Moreover, vacuolisation and oedema formation, as signs of acute pancreatitis, were also not detected. Similarly, serum levels of amylase, lipase, or glucose were not significantly difference in doxycycline treated versus untreated control mice. The observed histological alterations were confined to the pancreas; no significant changes were observed in the spleen, kidney, thymus or liver of treated animals (data not shown). After prolonged doxycycline administration for 8–12 weeks both the incidence and intensity of lymphocytic infiltrates increased.
Figure 5 Phenotypic changes of CMV‐rtTA/Luciferase‐tet(O)‐IKK(2)‐EE mice after doxycycline treatment. Double transgenic mice were left untreated (A) or stimulated with doxycycline for 4 (B), 8 (C) and 12 (D) weeks. Paraffin embedded pancreatic tissue sections were stained by hemalaun‐eosin and analysed by light microscopy. One representative section of 3–4 mice analysed per group is displayed.
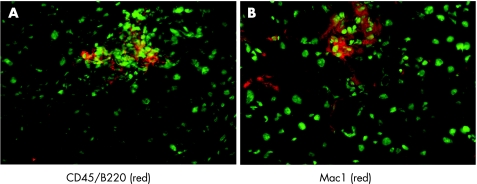
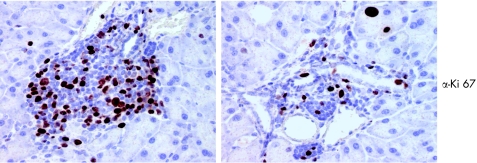
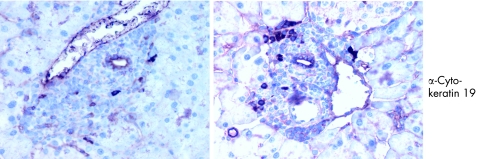
We further characterised the pancreatic infiltrates by examining cell composition. Immunofluorescence analysis revealed that infiltrations were positive for Mac1 and CD45/B220, cellular markers for macrophages and B lymphocytes (fig 6). We also performed immunohistochemistry on paraffin embedded sections. Figure 6A shows, as expected, that most of the infiltrating cells stain positive either for the B cell marker CD45 (B220) or for the macrophage marker Mac1 (fig 6B). We also detected few T lymphocytes compared with B cells and macrophages. These infiltrating T cells were positive for CD4 (fig 7B, C) but no CD8 positive T cells were observed in the pancreatic sections (fig 7D). For all of the above experiments, parallel studies on spleen sections drawn from the same animal were used as positive controls (table 1). Many of the infiltrating cells stained positive for Ki67, indicating that these cells were in a proliferative state (fig 8). Cellular proliferation was restricted to these sites of immune cell infiltration; analysis of pancreatic acinar cells showed only occasional staining for Ki67. Moreover, enhanced expression of adhesion molecules such as ICAM (CD54) and PECAM (CD31) were visualised on the endothelial cells of blood vessels in pancreatic sections whereas in the same sections CK19 expression was confined to the pancreatic ducts (fig 9, table 1).
Figure 6 Analysis of infiltrates. (A) Immunofluorescence analysis of frozen pancreatic sections stained with an antibody against B lymphocytes (CD45/B220; red fluorescence). The section was counterstained with green fluorescent Yopro to mark nuclei. (B) Staining of frozen pancreatic sections with an anti‐Mac1‐PE antibody staining for macrophages. Counterstaining for nuclei using green fluorescent Yopro was done on the same section.
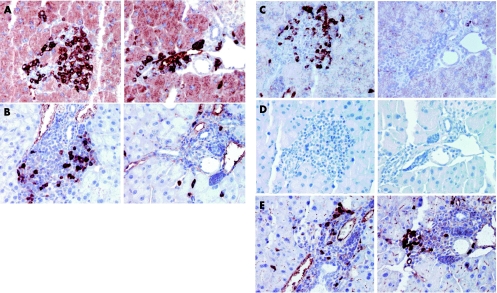
Figure 7 Characterisation of infiltrates. Staining of pancreatic tissue sections by immunohistochemistry using an antibody against B lymphocytes (CD45/B220 (A)) or T lymphocytes (anti‐CD3 (B)), as described in the methods section. Note that most cells stained positive for the B cell marker. (C, D) Characterisation of T cells. Paraffin embedded tissue sections were stained by immunohistochemistry using anti‐CD4 (C) and anti‐CD8 (D) antibodies. (E) Staining of pancreatic sections using an antibody against Mac1. Representative sections of two animals are shown in parallel.
Table 1 Immunohistochemistry of CMV‐IKK2 mice after 12 weeks of induction.
| Antibody | Target | Pancreas | Infiltrates | Lymph nodes | Spleen | |||
|---|---|---|---|---|---|---|---|---|
| Acini | Ducts | Vessels | ||||||
| Ki 67 | Ki 67 | (+) | + | − | +++ | +++ | ++ | Weakly increased proliferation of acini |
| CK19 | CK19 | − | ++ | − | + (Single cells) | − | − | |
| CD3 | CD3/T cells | − | − | − | + | ++ | ++ | |
| CD4 | CD4/T cells | − | − | − | + | ++ | ++ | |
| CD8 | CD8/T cells | − | − | − | − | + | + | |
| Mac1 | Macrophage | − | − | − | ++ | ++ | ++ | Diffuse in the tissue |
| CD45 | B cells | − | − | − | +++ | +++ | +++ | Diffuse staining in the tissue |
| CD54 | ICAM1 | − | − | + | − | nd | nd | Large vessels |
| CD144 | VE cadherin | − | − | + | − | nd | nd | Weak staining |
| CD31 | PECAM1 | − | − | + | − | nd | nd | |
Figure 8 Proliferation of immune cells in the pancreas. Anti‐Ki67 staining of paraffin embedded tissue sections by immunohistochemistry, as described in materials and methods. Positive signals indicate cellular proliferation. Note that a significant number of infiltrating cells show the proliferation marker Ki67.
Figure 9 Staining of pancreatic section using an antibody against cytokeratin 19.
Taken together, we showed that enhanced expression of constitutively active IKK2 in pancreatic acinar cells results in activation of the canonical NF‐κB pathway and subsequent infiltration of mainly B lymphocytes and macrophages. Furthermore, our data may suggest that factors are produced by pancreatic tissues that result in increased expression of adhesion molecules on vascular endothelium. As the expression of some of these factors is controlled by NF‐κB, we next examined the pancreatic specimens for NF‐κB dependent gene expression.
NF‐κB target gene expression is altered in the pancreas
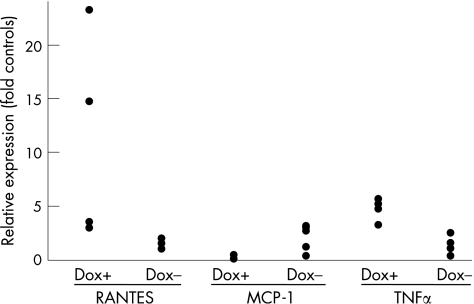
To analyse NF‐κB gene expression in our model we isolated RNA from acinar cells using laser capture microscopy to eliminate RNA contamination by the immune cell infiltrates and vascular tissues. With isolated mRNA, quantitative RT‐PCR was performed revealing increased levels of RANTES and TNFα in the acinar cells of doxycycline treated double transgenic animals (fig 10). Thus the active IKK2/NF‐κB pathway in pancreatic acinar cells leads to increased expression of RANTES and TNFα. This finding implies that that part of the cellular response could be a result of paracrine effects of secreted inflammatory cytokines.
Figure 10 mRNA expression of RANTES, MCP‐1 and tumour necrosis factor α (TNFα) in pancreatic acinar cells. Pancreatic acinar cells were isolated from frozen tissue sections by laser capture microdissection, as described in experimental procedures. RNA was extracted from acinar cells, amplified and subjected to the real‐time reverse transcription‐polymerase chain reaction analysis for expression of RANTES, MCP‐1 and TNFα. The results were normalised to expression of cyclophillin. Dox+, mice stimulated with doxycycline; Dox−, non‐stimulated mice. Each dot represents the measurement obtained in one animal normalised to expression of cyclophillin in the same animal.
Constitutive activation of the IKK complex influences caerulein pancreatitis
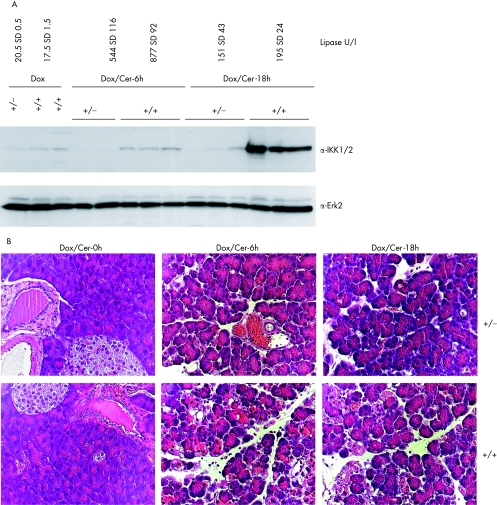
As mentioned above, induction of IKK2CA alone was not sufficient to induce changes comparable with experimental pancreatitis. To further define the role of IKK, we combined caerulein induced pancreatitis with induction of IKK2CA in acinar cells. Western blot analysis showed a moderate induction in IKK2CA protein expression in the pancreas of double transgenic mice treated with doxycycline for 10 weeks (fig 11A). Subsequent induction of experimental pancreatitis by caerulein injection caused a moderate increase in serum lipase levels (fig 11A). Surprisingly, application of caerulein also enhanced transgene (IKK2) expression, probably as a result of alteration of CMV promoter activity. Histological analysis revealed a clear increase in cellular vacuolisation, infiltration of leucocytes and oedema in the pancreas of mice overexpressing IKK2CA. The differences were remarkable as they were detectable as early as 6 hours after the first injection of caerulein and were still obvious after 18 hours (fig 11B). These data strongly suggest a critical role for the classical NF‐κB cascade in promoting experimental pancreatitis.
Figure 11 Analysis of secretagogue induced pancreatitis in CMV‐rtTA/Luciferase‐tet(O)‐IKK2‐EE mice after doxycycline treatment. (A) IKK2 expression in pancreatic extracts of mice stimulated with doxycycline (Dox) for 10 weeks; double transgenic mice (+/+) and single transgenic mice (+/−) were treated with supramaximal caerulein and sacrificed after 6 (Cer‐6h) or 18 h (Cer‐18h). Each group contains three animals. Detectable expression of IKK2 is only shown in double transgenic animals treated with doxycycline. The top panel displays mean (SD) values for serum lipase, measured in the indicated groups. (B) Double transgenic (+/+) or single transgenic (+/−)mice stimulated with doxycycline were left untreated (Dox/Cer‐0h) or stimulated with supramaximal caerulein doses and analysed after 6 (Dox/Cer‐6h) and 18 hours (Dox/Cer‐18). Paraffin embedded pancreatic tissue sections were stained by hemalaun‐eosin and analysed by light microscopy. One representative section of three animals analysed per group is displayed.
Discussion
This study describes a mouse model allowing conditional expression of IKK2CA in the pancreas. We used this model to investigate the effects of the NF‐κB signalling pathway on pancreas physiology, eliminating the side effects of NF‐κB‐activation on pancreatic development. In vive, administration of doxycycline to adult mice caused transgene expression. We found that expression of IKK2CA in pancreatic acinar cells induced the migration of B lymphocytes, macrophages and helper T cells to the pancreas. This effect was apparent as early as 4 weeks following first administration of doxycycline, and was monitored for up to 3 months.
To create our mouse model we crossed a mouse line where rtTA expression was driven by the CMV promoter25,26 with a mouse line that was transgenic for luciferase‐(tetO)7‐CA‐IKK2. Administration of doxycycline to the double transgenic animals results in a conformational change in the rtTA protein and allows for pancreas specific expression of the luciferase and IKK2CA proteins. We used luciferase expression as a sensitive method for testing the specificity of our system. Thus following 4 weeks of doxycycline treatment the double transgenic mice expressed high levels of luciferase in the pancreas with little or no enzyme present in the lung, brain, liver or lymphocytes. At this time point we observed elevated levels of luciferase in the kidney, thymus, stomach and muscle tissue but luciferase activity in these organs decreased to background levels after 8–12 weeks while activity in the pancreas increased. Levels of luciferase expression in the pancreas of double transgenic animals were 100‐fold greater than those observed in single transgenic mice, or in double transgenic mice which had not received doxycycline. Moreover, increased protein and kinase activity of IKK2 was only detected in the pancreatic tissue of double transgenic animals treated with doxycycline. These observations are supported by previously published results on the relative tissue specificity of the CMV promoter.31 Further analysis revealed that ectopic IKK2CA and luciferase are mainly expressed in pancreatic acinar cells. The mosaic expression pattern indicates that the CMV promoter activity does not have equal strength in all acinar cells. The patchy appearance may account for the relative moderate transgene expression and kinase activity of the endogenous IKK complex. Reduced biochemical activity of IKK2CA might be the result of a dilution effect as kinase activity is measured in whole tissue lysates. Nevertheless, IKK activity is enough to activate the canonical NF‐κB pathway, as shown by nuclear translocation of p65 (fig 4). Taken together, these results confirm our conditional animal model of pancreas specific IKK2CA expression. Interestingly, maximal expression of the IKK2 and luciferase transgenes was observed only after 8–12 weeks of continuous doxycycline administration. The reasons for this are unclear but may be the result of either the pharmacokinetics of doxycycline levels, cellular adaptation to the transgene or a combination of the two.
Continuous expression of IKK2CA in the pancreas results in the development of patchy cellular infiltrates. These infiltrates consist mainly of B lymphocytes and macrophages although some CD4+ T cells are also present. The localisation pattern of the infiltrating cells might be because of the uneven distribution of transgene expressions in the individual acinar cell rather than the result of paracrine cytokine stimulation. The infiltrates show reactivity with antibodies to Ki67, suggesting that the cells within the infiltrates are undergoing proliferation. In contrast, prolonged activation of the IKK complex did not induce hyperplasia or proliferation of the pancreatic cells. In our model system we also found that IKK2CA expression did not result in destruction, apoptosis or necrosis of pancreatic cells, nor did we detect any changes in the serum levels of amylase or lipase when compared with non‐stimulated mice. This is in part surprising, considering that active NF‐κB is thought to play a major role during induction of pancreatitis.32 This observation might be explained by comparing our model with secretagogue induced pancreatitis. The activation levels of the classical NF‐κB cascade, measured by staining for nuclear p65 in the pancreas of mice after IKK2CA induction, are considerably lower compared with those observed in caerulein induced acute pancreatitis (fig 4C). Although there are limitations to the method it suggests that the level of activation of the classical NF‐κB pathway in pancreatic acinar cells is crucial. The present study suggests however that the relatively low but consistent IKK activation is only sufficient to induce lymphocytic infiltrates into the pancreas and thus provides evidence for the molecular mechanisms of how pancreatic infiltrates are generated.
RNA extraction and real time PCR partly reveal the mechanisms by which IKK2CA induces infiltration of immune cells to the pancreas. We detected TNFα, RANTES and MCP‐1 in pancreatic lysates. Real time RT‐PCR reveals different sources for the cytokines: TNFα and RANTES mRNA are produced in pancreatic acini whereas the most likely source of MCP‐1 is infiltrating cells. The presence of TNFα makes autocrine or paracrine stimulation of pancreatic cells possible. This is supported by the observation that endothelial cells in small vessels stain positive for the TNFα regulated adhesion molecule ICAM (table 1). Acinar cells can secrete TNFα and RANTES in response to supramaximal cholecystokinin in vitro.33 Expression of these cytokines has been reported in several experimental models of pancreatitis indicating a general mechanism to induce cellular infiltrates to the pancreas. Interestingly, there are hints that inhibition of RANTES signalling might positively influence the course of acute pancreatitis in vivo.34,35
We observed in our model that doxycycline induces moderate IKK activity in pancreatic acinar cells with significant differences at the cellular level. The NF‐κB cascade is tightly regulated on different levels, and such regulation may provide an explanation for the apparent discrepancies in the consequences of NF‐κB activation in acute pancreatitis. Induction of massive NF‐κB activation in the pancreas by adenoviral mediated transfer of RelA leads to rapid destruction of pancreatic tissue.32 In our model, moderate or weak activation of the IKK complex was associated only with lymphocytic infiltrates. This appears to be a gradual process and may point to different functions of the activity levels of the IKK complex and NF‐κB proteins in inflammatory diseases of the pancreas. This is supported by the fact that knockout models reveal distinct phenotypes depending on which member of the NF‐κB cascade is inactivated.36
In human autoimmune pancreatitis (AIP), recently described as a new clinical entity, the main histopathological criterion is a periductal lymphoplasmacytic infiltrate often located in the head of the pancreas.37,38,39 Importantly, the absence of focal autodigestive necrosis distinguishes the disease from other forms of pancreatitis, such as alcoholic chronic pancreatitis.39,40,41,42 The pathophysiology of the disease is unclear but there is an overlap of autoimmune pancreatitis with other autoimmune diseases.43 With regard to AIP, the mosaic pattern of IKK2CA expression might account for the similarities in patchy leucocytic infiltration. These morphological similarities do not establish a link between our mouse model and AIP but our findings are suggestive of a role for IKK complex activation in human AIP.
If moderate activation of IKK2 in the pancreas is only sufficient to induce lymphocytic infiltrations, it would be interesting to observe the consequences of ectopic IKK complex activation in acute experimental pancreatitis. Our data suggest that even weak activity of the IKK complex is sufficient to worsen the course of disease. These observations support the hypothesis that activation of the NF‐κB cascade in acinar cells promotes the course of experimental pancreatitis via induction of cytokines and/or increased lymphocytic infiltration. Moreover, the data underline the hypothesis that the NF‐?κB cascade is regulated on many different levels so that little changes in the activation pattern result in major consequences in inflammatory diseases.
In the present study we have provided the first experimental model where IKK2CA can be conditionally expressed in pancreatic acinar cells. We showed with this model that moderate expression of IKK2CA is only sufficient to induce an inflammatory reaction in vivo but does not lead to induction of pancreatitis. In contrast, a combination of IKK2CA with caerulein induced pancreatitis promotes disease activity. In addition, our model provides long lasting expression of IKK2CA and may be useful for studies of chronic diseases such as tumour promotion in chronic inflammatory states.
Acknowledgements
We thank L Federov and UR Rapp for providing the CMV‐rtTA transgenic mice. We also thank J Slupsky and T Seufferlein for critical reading of the manuscript; B Knobel, U Möhnle and U Leschik for excellent technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 518 to TW and CKW).
Abbreviations
AIP - autoimmune pancreatitis
IKK2 - IκB kinase‐2
NF‐κB - nuclear factor κB
RT‐PCR - reverse transcription‐polymerase chain reaction
TNFα - tumour necrosis factor α
Footnotes
Competing Interest: None declared.
References
- 1.Rothwarf D M, Karin M. The NF‐kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. Sci STKE 19991999RE1. [DOI] [PubMed] [Google Scholar]
- 2.Senftleben U, Karin M. The IKK/NF‐kappaB pathway. Crit Care Med 200230S18–S26. [PubMed] [Google Scholar]
- 3.Israel A. The IKK complex: an integrator of all signals that activate NF‐kappaB? Trends Cell Biol 200010129–133. [DOI] [PubMed] [Google Scholar]
- 4.Mercurio F, Zhu H, Murray B W.et al IKK‐1 and IKK‐2: cytokine‐activated IkappaB kinases essential for NF‐kappaB activation. Science 1997278860–866. [DOI] [PubMed] [Google Scholar]
- 5.Ducut Sigala J L, Bottero V, Young D B.et al Activation of transcription factor NF‐kappaB requires ELKS, an IkappaB kinase regulatory subunit. Science 20043041963–1967. [DOI] [PubMed] [Google Scholar]
- 6.Chen L W, Egan L, Li Z W.et al The two faces of IKK and NF‐kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia–reperfusion. Nat Med 20039575–581. [DOI] [PubMed] [Google Scholar]
- 7.DiDonato J, Mercurio F, Rosette C.et al Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol 1996161295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiDonato J A. IKK alpha on center stage. Sci STKE 20012001E1. [DOI] [PubMed] [Google Scholar]
- 9.Senftleben U, Cao Y, Xiao G.et al Activation by IKKalpha of a second, evolutionary conserved, NF‐kappa B signaling pathway. Science 20012931495–1499. [DOI] [PubMed] [Google Scholar]
- 10.Steinle A U, Weidenbach H, Wagner M.et al NF‐kappaB/Rel activation in caerulein pancreatitis. Gastroenterology 1999116420–430. [DOI] [PubMed] [Google Scholar]
- 11.Tando Y, Algul H, Wagner M.et al Caerulein‐induced NF‐kappaB/Rel activation requires both Ca2+ and protein kinase C as messengers. Am J Physiol 1999277G678–G686. [DOI] [PubMed] [Google Scholar]
- 12.Tando Y, Algul H, Schneider G.et al Induction of IkappaB‐kinase by cholecystokinin is mediated by trypsinogen activation in rat pancreatic lobules. Digestion 200266237–245. [DOI] [PubMed] [Google Scholar]
- 13.Schmid R M, Adler G. NF‐kappaB/rel/IkappaB: implications in gastrointestinal diseases. Gastroenterology 20001181208–1228. [DOI] [PubMed] [Google Scholar]
- 14.Gukovsky I, Gukovskaya A S, Blinman T A.et al Early NF‐kappaB activation is associated with hormone‐induced pancreatitis. Am J Physiol 1998275G1402–G1414. [DOI] [PubMed] [Google Scholar]
- 15.Altavilla D, Famulari C, Passaniti M.et al Attenuated caerulein‐induced pancreatitis in nuclear factor‐kappaB‐deficient mice. Lab Invest 2003831723–1732. [DOI] [PubMed] [Google Scholar]
- 16.Weber C K, Liptay S, Wirth T.et al Suppression of NF‐kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology 20001191209–1218. [DOI] [PubMed] [Google Scholar]
- 17.Bantel H, Berg C, Vieth M.et al Mesalazine inhibits activation of transcription factor NF‐kappaB in inflamed mucosa of patients with ulcerative colitis. Am J Gastroenterol 2000953452–3457. [DOI] [PubMed] [Google Scholar]
- 18.Egan L J, Eckmann L, Greten F R.et al IkappaB‐kinase beta‐dependent NF‐kappaB activation provides radioprotection to the intestinal epithelium. Proc Natl Acad Sci U S A 20041012452–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gukovskaya A S, Hosseini S, Satoh A.et al Ethanol differentially regulates NF‐kappaB activation in pancreatic acinar cells through calcium and protein kinase C pathways. Am J Physiol Gastrointest Liver Physiol 2004286G204–G213. [DOI] [PubMed] [Google Scholar]
- 20.Pandol S J, Gukovsky I, Satoh A.et al Animal and in vitro models of alcoholic pancreatitis: role of cholecystokinin. Pancreas 200327297–300. [DOI] [PubMed] [Google Scholar]
- 21.Baron U, Bujard H. Tet repressor‐based system for regulated gene expression in eukaryotic cells: principles and advances. Methods Enzymol 2000327401–421. [DOI] [PubMed] [Google Scholar]
- 22.Baron U, Gossen M, Bujard H. Tetracycline‐controlled transcription in eukaryotes: novel transactivators with graded transactivation potential. Nucleic Acids Res 1997252723–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urlinger S, Baron U, Thellmann M.et al Exploring the sequence space for tetracycline‐dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci U S A 2000977963–7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayford M, Bach M E, Huang Y Y.et al Control of memory formation through regulated expression of a CaMKII transgene. Science 19962741678–1683. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann O, Baumann B, de Lorenzi R.et al IKK mediates ischemia‐induced neuronal death. Nat Med 2005111322–1329. [DOI] [PubMed] [Google Scholar]
- 26.Kistner A, Gossen M, Zimmermann F.et al Doxycycline‐mediated quantitative and tissue‐specific control of gene expression in transgenic mice. Proc Natl Acad Sci U S A 19969310933–10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumann B, Weber C K, Troppmair J.et al Raf induces NF‐kappaB by membrane shuttle kinase MEKK1, a signaling pathway critical for transformation. Proc Natl Acad Sci U S A 2000974615–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber C K, Slupsky J R, Kalmes H A.et al Active Ras induces heterodimerization of cRaf and BRaf. Cancer Res 2001613595–3598. [PubMed] [Google Scholar]
- 29.Wagner M, Weber C K, Bressau F.et al Transgenic overexpression of amphiregulin induces a mitogenic response selectively in pancreatic duct cells. Gastroenterology 20021221898–1912. [DOI] [PubMed] [Google Scholar]
- 30.Wagner M, Greten F R, Weber C K.et al A murine tumor progression model for pancreatic cancer recapitulating the genetic alterations of the human disease. Genes Dev 200115286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhan Y, Brady J L, Johnston J M.et al Predominant transgene expression in exocrine pancreas directed by the CMV promoter. DNA Cell Biol 200019639–645. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Ji B, Ernst S A.et al NF‐kB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology 2002122448–457. [DOI] [PubMed] [Google Scholar]
- 33.Yang B M, Demaine A G, Kingsnorth A. Chemokines MCP‐1 and RANTES in isolated rat pancreatic acinar cells treated with CCK and ethanol in vitro. Pancreas 20002122–31. [DOI] [PubMed] [Google Scholar]
- 34.Gerard C, Frossard J L, Bhatia M.et al Targeted disruption of the beta‐chemokine receptor CCR1 protects against pancreatitis‐associated lung injury. J Clin Invest 19971002022–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatia M, Proudfoot A E, Wells T N.et al Treatment with Met‐RANTES reduces lung injury in caerulein‐induced pancreatitis. Br J Surg 200390698–704. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh S, Karin M. Missing pieces in the NF‐kB puzzle. Cell 2002109S81–S96. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida K, Toki F, Takeuchi T.et al Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci 1995401561–1568. [DOI] [PubMed] [Google Scholar]
- 38.Ammann R W, Heitz P U, Kloppel G. The “two‐hit” pathogenetic concept of chronic pancreatitis. Int J Pancreatol 199925251. [PubMed] [Google Scholar]
- 39.Kloppel G, Luttges J, Lohr M.et al Autoimmune pancreatitis: pathological, clinical, and immunological features. Pancreas 20032714–19. [DOI] [PubMed] [Google Scholar]
- 40.Ectors N, Maillet B, Aerts R.et al Non‐alcoholic duct destructive chronic pancreatitis. Gut 199741263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamano H, Kawa S, Horiuchi A.et al High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001344732–738. [DOI] [PubMed] [Google Scholar]
- 42.Okazaki K, Uchida K, Ohana M.et al Autoimmune‐related pancreatitis is associated with autoantibodies and a Th1/Th2‐type cellular immune response. Gastroenterology 2000118573–581. [DOI] [PubMed] [Google Scholar]
- 43.Okazaki K, Chiba T. Autoimmune related pancreatitis. Gut 2002511–4. [DOI] [PMC free article] [PubMed] [Google Scholar]