Interleukin1β (IL1β) is upregulated in the presence of Helicobacter pylori infection.1 IL1β polymorphisms with T/T and T/C genotypes enhance IL1β production, and are associated with an increased risk of H pylori‐induced hypochlorhydria2 and gastric cancer.3 The relationship between H pylori and CpG island methylation has been repeatedly reported.4,5,6 It has been reported that IL1β can modulate CpG island methylation through activation of DNA methyltransferase and hence repress gene expression.7 We therefore hypothesised that patients with H pylori infection and IL1 polymorphism, by the production of IL1β, are predisposed to gastric cancer development through the CpG island methylation pathway.
We obtained surgical specimens and their corresponding peripheral blood from 98 consecutive patients with gastric cancer admitted to Queen Mary Hospital, Hong Kong. This study was approved by the ethics committee. The methylation status of the death‐associated protein‐kinase, O6‐methyl‐guanine methyltransferase, p16 genes8 and E‐cadherin9 promoter was determined by methylation‐specific polymerase chain reaction. Genotyping of IL1 polymorphism was performed as reported previously.2,10H pylori status was determined by serology using a commercially available ELISA kit (pylori DTect ELISA, Diagnostic Technology Pty, New South Wales, Australia) and histology using Giemsa stain. H pylori status was considered to be positive if either test showed a positive result.
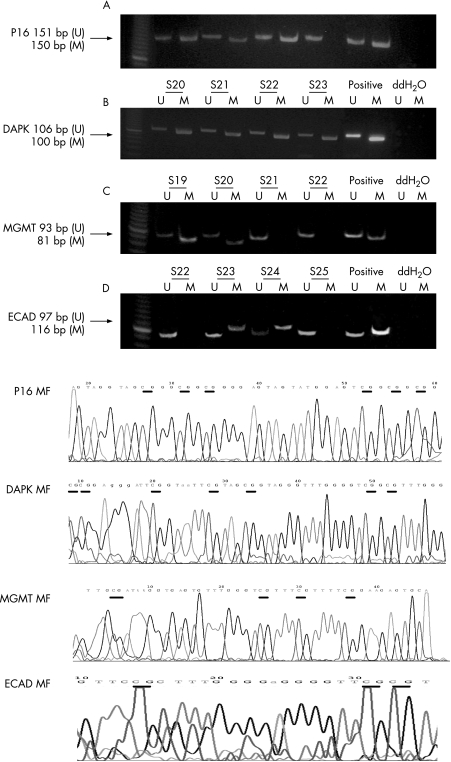
H pylori infection was present in 65% (64) of patients. In the IL1β gene, the T and C alleles at the ‐511 locus of the IL1β gene were in near total linkage disequilibrium with the C and T alleles at the ‐31 locus. Analysis was therefore restricted to the IL1β‐511 locus, but the associations with the ‐31 locus were identical. The allele frequencies at IL1β‐511 were 16% (16), 59% (58) and 25% (24) for C/C, C/T and T/T genotypes, respectively. Methylation of methylguanine‐DNA methyltransferase, E‐cadherin, death‐associated protein and p16 was present in 30% (29), 59% (58), 43% (42) and 45% (44) of patients, respectively (examples shown in fig 1). Patients with the T/T or T/C genotype showed an increased odds ratio (OR) of 6.4 and 3.7, respectively, with respect to the C/C genotype for developing methylation at two or more genes (table 1). This OR for developing methylation at two or more genes was markedly increased in patients with H pylori infection and with the T/T (12.5) or T/C (5.6) genotype with respect to the C/C genotype but was not present in patients without H pylori infection. However, no association of the genotype on an individual methylation marker was seen (table 1). Also, there was no association between the number of genes methylated and H pylori status, no difference in age between those with or without methylation at two or more genes, and anatomical site, except that lower stages (stages I and II) were associated with more frequent methylation at two or more genes (table 1).
Figure 1 Examples of polymerase chain reaction products of P16, DAPK, E‐cadherin (ECAD) and O2‐methyl‐guanine methyl transferase (MGMT) genes. The lower panel shows segments of the methylated sequences of these gene products. Methylated cytosine residues that are unchanged are shown as underlined.
Table 1 Correlation between methylation at two or more genes and IL1β‐511 genotype, and other tumour characteristics.
| Less than two genes methylated | Two or more genes methylated | OR | 95% CI | |
|---|---|---|---|---|
| Locus at IL1β‐511 (whole group, n = 98), overall p = 0.02 | ||||
| C/C | 56.2% (9) | 43.8% (7) | 1 | |
| C/T | 25.9% (15) | 74.1% (43) | 3.7 | 1.2 to 12.1 |
| T/T | 16.7% (4) | 83.3% (20) | 6.4 | 1.6 to 30.7 |
| Locus at IL1β‐511 (H pylori positive, n = 64) overall p = 0.03 | ||||
| C/C | 62.5% (5) | 37.5% (3) | 1 | |
| C/T | 23.1% (9) | 76.9% (30) | 5.6 | 1.2 to 31.7 |
| T/T | 11.8% (2) | 88.2% (15) | 12.5 | 1.8 to 125.7 |
| Locus at IL1β‐511 (H pylori negative, n = 34), overall p = 0.61 | ||||
| C/C | 50% (4) | 50% (4) | 1 | |
| C/T | 31.6% (6) | 68.4% (13) | 2.2 | 0.4 to 12.4 |
| T/T | 28.6% (2) | 71.4% (5) | 2.5 | 0.3 to 25.9 |
| H pylori status, overall p = 0.29 | ||||
| + | 25% (16) | 75% (48) | 1.6 | 0.7 to 4 |
| − | 35% (12) | 65% (22) | 1 | |
| Patient age (years), overall p = 0.44 | ||||
| ⩽60 | 23% (7) | 77% (23) | 1 | |
| >60 | 31% (21) | 69% (47) | 0.7 | 0.2 to 1.7 |
| Tumour stage, overall p = 0.004 | ||||
| I and II | 12.8% (5) | 87.2% (34) | ||
| III and IV | 39% (23) | 61% (36) | 0.23 | 0.07 to 0.63 |
| Anatomical site, overall p = 0.55 | ||||
| Antrum | 27% (20) | 73% (54) | 1 | |
| Non‐antrum | 33.3% (8) | 66.7% (16) | 0.7 | 0.3 to 2 |
| Histology (Lauren's classification), overall p = 0.09 | ||||
| Intestinal | 21.2% (11) | 78.8% (42) | 1 | |
| Diffuse | 32.3% (10) | 67.7% (21) | 0.55 | 0.2 to 1.5 |
| Mixed | 50.0% (7) | 50.0% (7) | 0.26 | 0.1 to 0.9 |
| Methylation status stratified by genotype | ||||
| H pylori positive | H pylori negative | |||
| C/C | 62.5% (5/8) | 50% (4/8) | 0.6 | 0.07 to 4.4 (p = 0.61) |
| C/T | 23% (9/39) | 68% (13/19) | 1.5 | 0.4 to 5.2 (p = 0.49) |
| T/T | 11.7% (2/17) | 71% (5/7) | 3 | 0.3 to 31.1 (p = 0.33) |
*ORs of two or more genes methylated, with the first group being the referent group.
The underlying mechanisms or the environmental factors governing the simultaneous methylation of multiple genes in gastric cancer are still unclear. Hmadcha et al7 have reported gene silencing due to methylation at the CpG island in the presence of IL1β. We have previously reported that eradication of H pylori may result in reversal of methylation at tumour suppressor genes in non‐lesional gastric mucosa.5 Our study may further support the idea that H pylori infection stimulates the production of IL1β, and the presence of the proinflammatory T allele further enhances the production of IL1β. The synergistic effect of these two factors was observed in our study to correlate with the increased frequency of methylated genes. Thus, patients with H pylori infection and IL1β‐511 T/T genotype may be predisposed to gastric cancer through the CpG island methylation pathway.
Acknowledgements
This work was supported by the Gastrointestinal Cancer Research Fund, University of Hong Kong.
Footnotes
Competing interests: None.
References
- 1.Yamaoka Y, Kita M, Kodama T.et al Induction of various cytokines and development of severe mucosal inflammation by cag A gene positive Helicobacter pylori strains. Gut 199741442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furuta T, El‐Omar E M, Xiao F.et al Interleukin 1beta polymorphisms increase risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology 200212392–105. [DOI] [PubMed] [Google Scholar]
- 3.El‐Omar E M, Carrington M, Chow W H.et al Interleukin‐1 polymorphisms associated with increased risk of gastric cancer. Nature 2000404398–402. [DOI] [PubMed] [Google Scholar]
- 4.Chan A O, Lam S K, Wong B C.et al Promoter methylation of E‐cadherin gene in gastric mucosa associated with Helicobacter pylori infection and in gastric cancer. Gut 200352502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan A O, Peng J Z, Lam S K.et al Disappearing of E‐cadherin promoter hypermethylation status after Helicobacter pylori eradication in patients with chronic gastritis. Gut 200655463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maekita T, Nakazawa K, Mihara M.et al High levels of aberrant DNA methylation in Helicobacter pylori‐infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res 200612989–995. [DOI] [PubMed] [Google Scholar]
- 7.Hmadcha A, Bedoya F J, Sobrino F.et al Methylation‐dependent gene silencing induced by interleukin 1beta via nitric oxide production. J Exp Med 19991901595–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang G H, Lee H J, Hwang K S.et al Aberrant CpG island hypermethylation of chronic gastritis, in relation to aging, gender, intestinal metaplasia, and chronic inflammation. Am J Pathol 20031631551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman J G, Graff J R, Myohanen S.et al Methylation‐specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A 1996939821–9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarlow J K, Blakemore A I, Lennard A.et al Polymorphism in human IL‐1 receptor antagonist gene intron 2 is caused by variable numbers of an 86‐bptandem repeat. Hum Genet 19939403–404. [DOI] [PubMed] [Google Scholar]