Abstract
Background and aim
Chronic pancreatitis is characterised by severe abdominal neuropathic pain, perineural inflammatory cell infiltrations and intrapancreatic neural growth. Artemin was recently shown to eliminate neuropathic pain and reverse neurochemical damage after nerve injury. The role of artemin and its receptor GFRα3 was investigated in patients with chronic pancreatitis.
Methods
Expression of artemin and its receptor GFRα3 was studied in chronic pancreatitis (n = 66) and normal (n = 22) pancreatic tissues by quantitative reverse transcription‐polymerase chain reaction (QRT‐PCR) and western blot analysis. Artemin expression was correlated with pain and pathomorphological changes (inflammation, perineural inflammatory cell infiltration, neural alterations and fibrosis). Immunohistochemistry was used to localise artemin and GFRα3 in the tissues. To detect sources of artemin, primary human pancreatic stellate cells (hPSCs) were isolated and analysed by QRT‐PCR and immunocytology analysis.
Results
In chronic pancreatitis, artemin and GFRα3 were significantly overexpressed and located in smooth muscle cells of arteries, Schwann cells and neural ganglia. Increased levels of artemin mRNA correlated with pain severity, inflammation, perineural inflammatory cell infiltration, neural density and hypertrophy. Furthermore, the severity of fibrosis was positively related with artemin expression and neural alterations. Activated hPSCs expressed low basal levels of artemin mRNA which were upregulated by exposure to transforming growth factor (TGF)β1.
Conclusions
Overexpression of artemin in chronic pancreatitis might function as a compensatory upregulation in order to repair neural damage incurred by ongoing pancreatic inflammation. Upregulation of TGFβ1 seems not only to increase pancreatic fibrosis but also to contribute to neural alteration by stimulating artemin expression in hPSCs. However, overexpression of endogenous artemin does not seem to be sufficient to prevent pain in chronic pancreatitis.
Chronic pancreatitis is an inflammatory disease characterised by irreversible and progressive destruction of the whole organ, resulting in acinar cell damage and replacement of the lost parenchyma by dense fibrotic tissue.1,2,3 The cardinal symptom of chronic pancreatitis is excruciating abdominal pain. However, the precise mechanisms of pain generation and perpetuation in chronic pancreatitis remain unknown. One hypothesis is that increased intraductal pressure caused by pancreatic fibrosis via strictures, interstitial hypertension, pancreatic ischaemia and pancreatic pseudocysts may contribute to the generation of pain in chronic pancreatitis.4 Another theory, that altered intrapancreatic nerves trigger pain in chronic pancreatitis, is based on the fact that histological analysis of specimens from patients with well developed chronic pancreatitis demonstrated prominent intrapancreatic nerves embedded in the densely fibrotic stroma.5 The nerves in chronic pancreatitis, which are enlarged and increased in number, are more prominent than in the normal pancreas.5,6 The perineural sheaths of the nerves demonstrate severe ultrastructural changes, and are harmed in such a way that they no longer provide a protective barrier between the surrounding connective tissue and the internal neural components.5 Because of the loss of the protective barrier, the nerves become more susceptible to noxious substances that are present in the extracellular matrix. In addition, lymphocytes can easily infiltrate the intrapancreatic nerves and cause a local pancreatitis associated neuritis which is directly correlated to pain in patients with chronic pancreatitis.4,7,8 As a result of the direct injury of the intrapancreatic nerves in chronic pancreatitis, the resulting pain is described as neuropathic pain.9 It is known that alterations of intrapancreatic nerves and the severity of pain are directly associated with enhanced expression of the growth associated protein 43, which is a marker for neuronal growth and plasticity.8,10 Brain derived neurotrophic factor, nerve growth factor and high affinity receptor for nerve growth factor, TrkA, are also upregulated11,12 in metaplastic ductal cells and intrapancreatic ganglia in chronic pancreatitis. TrkA and brain derived neurotrophic factor are predominantly expressed in the perineurium of enlarged pancreatic nerves and are directly correlated with pain frequency and intensity, indicating that neurotrophic factors are involved in neural changes and pain generation in patients with chronic pancreatitis.11,12
Artemin is a novel neurotrophic factor which could be a possible mediator of such neural changes and pain perception in chronic pancreatitis. It belongs to the glial derived neurotrophic factor (GDNF) family of ligands which consists of four members: GDNF, neurturin,13 persephin14 and artemin.15,16 All four are members of the transforming growth factor (TGF)β superfamily, presenting different neurotrophic properties.16 There is up to 50% amino acid sequence homology between the members of the GDNF family and TGFβ.17 The signalling specificity of artemin is achieved by the initial binding to the GDNF family receptor (GFR)α3.18,19,20,21 Subsequent signalling is then mediated by the receptor tyrosine kinase RET,18,22 with downstream targets in the Ras/ERK, PI3K/AKT, p38/MAPK and JNK pathways affecting cell growth, differentiation and survival.18,22 To date, artemin expression has been demonstrated in the brain, in dorsal root ganglia, in Schwann cells and in vascular smooth muscle cells.16,19,23 It enhances survival, proliferation and regeneration of neurons, and increases the neuronal density and neurite outgrowth of sympathetic neurons in vitro.16,19,24 In addition, artemin acts as a guidance molecule that induces migration and axonal projection from sympathetic neurons by signalling through GFRα3/RET complexes.23 Artemin is also involved in nociception. It reduces experimental neuropathic pain and reverses neural damage caused by spinal nerve ligation.25
Assuming that pain in chronic pancreatitis is of neuropathic origin, it is tempting to consider a therapeutic application of artemin in chronic pancreatitis. Therefore, in this study, we investigated the association of artemin with pain, and neural and non‐neural pathomorphological changes in patients with chronic pancreatitis.
Materials and methods
Patients and tissues
Sixty six chronic pancreatitis tissue samples were collected from patients undergoing pancreatic head resection (49 males, 17 females; median age 45 years). The aetiology of the pancreatitis was alcoholic chronic pancreatitis (n = 30), biliary chronic pancreatitis (n = 8), idiopathic chronic pancreatitis (n = 22) and autoimmune pancreatitis (n = 6). Written informed consent was obtained from all patients. Normal pancreatic tissue samples were collected from healthy organ donors when there was no appropriate recipient for transplantation (n = 22; 14 males, eight females; median age 41 years). The study was approved by the ethics committees of the University of Bern, Switzerland, and the University of Heidelberg, Germany.
Resected pancreatic tissues were divided into several parts and the aliquots were (a) frozen in liquid nitrogen and stored at −80°C for protein extraction, (b) taken into RNA‐later (Ambion, Huntington, UK) for RNA extraction, (c) fixed in 5% paraformaldehyde, and later embedded in paraffin for immunohistochemical analysis and (d) immediately processed for isolation of human pancreatic stellate cells (hPSC).
Reagents
The following reagents were purchased: RPMI‐1640, Dulbecco's modified Eagle's medium, trypsin‐EDTA and penicillin‐streptomycin from Invitrogen (Karlsruhe, Germany); fetal calf serum (FCS) from PAN Biotech (Aidenbach, Germany); artemin and GFRα3 rabbit polyclonal antibodies from Abcam (Cambridge, UK); PGP9.5 mouse monoclonal antibody from Biotech (Aidenbach, Germany); α‐SMA mouse monoclonal antibody from Dako Cytomation (Hamburg, Germany); FluoroLink Cy3 labelled goat anti‐mouse IgG antibody and FITC anti‐rabbit IgG, HRPO linked antibodies and ECL immunoblotting detection reagents, from Amersham Biosciences (Buckinghamshire, UK). Dako Envision system (Hamburg, Germany) was used for immunohistochemistry. Recombinant human fibroblast growth factor (FGF)‐I (232‐FA) and FGF‐II (234‐FSE), and recombinant human platelet derived growth factor (PDGF)‐AA (221‐AA) and PDGF‐BB (220‐BB) were purchased from R&D Systems (Wiesbaden, Germany). Recombinant human TGFβ1 (GF111) was purchased from Chemicon (Temecula, California, USA). All other chemicals were from Sigma Chemical Company (Taufkirchen, Germany).
Real time light cycler quantitative reverse transcription‐polymerase chain reaction (QRT‐PCR)
All reagents and equipment for mRNA/cDNA preparation were purchased from Roche Applied Science (Mannheim, Germany). Extraction of mRNA from normal and chronic pancreatitis tissues was performed by automated isolation using the MagNA Pure LC instrument and isolation kits I (for cells) and II (for tissue). cDNA was prepared using the first strand cDNA synthesis kit for RT‐PCR according to the manufacturer's instructions. Real time PCR was performed with the Light Cycler Fast Start DNA SYBR Green kit. All primers were obtained from Search‐LC (Heidelberg, Germany). The calculated number of specific transcripts was normalised to the housekeeping genes cyclophilin B and hypoxanthine guanine phosphoribosyltransferase and expressed as copy number (transcript) per μl of input cDNA, as described previously.26
Western blot analysis
Protein extraction and western blot analysis of cell culture monolayers or tissues were performed as described previously.27 Protein (30 μg/well) was separated and electroblotted, and the membrane was exposed to artemin and GFRα3 antibodies (dilution of 1:500) at 4°C overnight. Signal detection was performed using an enhanced chemiluminescence reaction. After film scanning, densitometric analysis was performed using the ImageJ software (NIH, USA). Specific signal intensity was calculated and presented as a fold increase over background, as published previously.28 Equal loading was assured by stripping the blots and reprobing with anti‐γ‐tubulin antibodies (Santa Cruz Biotechnologies, Heidelberg, Germany).
Immunohistochemistry
Paraffin embedded tissue sections of normal pancreas and chronic pancreatitis samples were analysed by immunostaining using the Dako Envision system with DAB as chromogen, as described previously.27 PGP9.5, artemin and GFRα3 antibodies were used at a dilution of 1:100. Non‐immunised mouse and rabbit IgG antibodies (Dako, Hamburg, Germany) were used as negative controls. Digital imaging was performed using the Zeiss AxioCam HR system (Carl Zeiss AG, Oberkochen, Germany).
Pathomorphological analysis
Briefly, 3 μm thick sections, obtained from paraformaldehyde fixed and paraffin embedded pancreatic tissue, were stained with haematoxylin–eosin. Histopathological analysis was performed by two independent observers blinded to patient diagnosis and QRT‐PCR data, followed by resolution of any differences by joint review and consultation with a third observer. This histomorphological evaluation of the specimen included: the severity of fibrosis, the severity of inflammation, the activity of inflammation, and the severity and activity of the perineural inflammation.
The severity of fibrosis was evaluated as described by Ammann and colleagues29 using a scoring system based on focal versus diffuse extension of intralobular and perilobular fibrosis. The severity and distribution of fibrosis in the investigated specimens were graded according to a scoring system shown in table 1. Severity of fibrosis was determined by addition of intralobular and perilobular fibrosis scores of: mild 0 (0–4), moderate I (5–9) or severe II (10–12) fibrosis.
Table 1 Scoring system for the evaluation of fibrosis in chronic pancreatitis.
| Severity of fibrosis | ||||
|---|---|---|---|---|
| Mild | Moderate | Severe | Score | |
| Perilobular | ||||
| Focal | 1 | 2 | 3 | 1–3 |
| Diffuse | 4 | 5 | 6 | 4–6 |
| Intralobular | ||||
| Focal | 1 | 2 | 3 | 1–3 |
| Diffuse | 4 | 5 | 6 | 4–6 |
The severity of inflammation was scored as absent (0), mild (I), moderate (II) or severe (III), based on the determination of the overall accumulation of inflammatory cells (lymphocytes, plasma cells, macrophages). The activity of inflammation was based on the presence and density of neutrophil granulocytes representing the early inflammatory response. These were scored as absent (0), mild (I) or moderate to severe (II).
In addition to the overall assessment of inflammation, the severity and activity of inflammation were separately determined in intrapancreatic nerves and their direct surroundings. The severity of perineural inflammation was scored as absent (0), perineural (score I) or intraneural (score II). Determination of the activity of perineural inflammation was based on the presence and density of neutrophil granulocytes, and was scored as absent (0), mild (I) or moderate to severe (II)
Pain scoring
In all patients with chronic pancreatitis, the individual pain score was registered prior to the operation according to pain intensity and frequency, as described previously.30 The intensity of pain was graded using the scale: 0 = none, 1 = mild, 2 = moderate and 3 = strong pain. The frequency of pain was graded as 1 = monthly, 2 = weekly and 3 = daily. To calculate pain severity, pain intensity and frequency were multiplied. According to the final pain score, the patients were divided into three groups: pain 0 (0), representing the group of patients who did not have any pain; pain I (1–3), representing the group of patients who suffered from mild pain; and pain II (4–9), the group of patients with moderate to severe pain.
Quantitative analysis of nerve number and the total nerve area in chronic pancreatitis
Intrapancreatic nerves in tissue sections of normal pancreas and patients with chronic pancreatitis were immunostained using the pan‐neuronal marker PGP9.5.31,32 To quantify the total number of nerves (neural density) and the total nerve area (neural hypertrophy, NHY), the entire pancreatic tissue sections were scanned and reconstructed into a mosaic image using the Zeiss KS300 program. In every microscopic cut‐out of the mosaic, the PGP9.5 immunoreactive nerves were circumscribed. The number of nerves and nerve area in the entire area of the investigated tissue sections were assessed with the Zeiss KS300 program. The number of nerves was expressed as the total number of nerves within the total area (mm2) of the investigated specimen. Accordingly, the total nerve area was expressed as the ratio of the measured area of the nerves to the total area of the analysed section.
Isolation of human pancreatic stellate cells
Human pancreatic stellate cells (hPSCs) were isolated from freshly resected pancreatic tissue of patients with chronic pancreatitis using the outgrowth method described by Bachem et al.33 hPSCs were cultivated in a 1:1 dilution of Ham's F‐12 nutrient medium and low glucose Dulbecco's modified Eagle's medium supplemented with penicillin, streptomycin, amphotericin and 10% FCS. Double immunofluorescence–immunocytology was performed as described previously.34 Incubations with the primary mouse monoclonal antibodies α‐SMA (1:100) and polyclonal rabbit artemin (1:100), and GFRα3 (1:100) were performed at room temperature for 40 min. As secondary antibodies, anti‐mouse‐Cy3 and anti‐rabbit‐FITC were used.
Growth factor stimulation of hPSCs
hPSCs were grown in 10 cm Petri dishes and six well plates for protein and RNA extraction, respectively. When the cells reached 70% confluence, fresh medium supplemented with 1% FCS containing growth factors at their ED50 doses was applied for 48 h. PBS was used as a control. The ED50 concentrations were as follows: FGF‐I 0.25 ng/ml (in combination with 10 μg/ml heparin); FGF‐II 0.3 ng/ml; PDGF‐AA 4 ng/ml; PDGF‐BB 2 ng/ml; and TGFβ1 5 ng/ml. All experiments were repeated twice.
Statistical analysis
Statistical analysis was performed using the SAS software (release 9.1; SAS Institute Inc., Cary, North Carolina, USA). Non‐parametric statistical methods were used to analyse mRNA levels of artemin and GFRα3, judged by the Shapiro–Wilk test. Results are expressed as median with interquartile range (IQR Q1–Q3). Comparisons of intrapancreatic artemin and GFRα3 expression in chronic pancreatitis samples with normal control patients were performed using the Mann–Whitney U test. To compare more than two subgroups of samples with respect to artemin and GFRα3 in chronic pancreatitis samples, the Kruskal–Wallis test was used. If a significant result was found, further pairwise comparisons of the subgroups were performed using the Mann–Whitney U test. The relationship between artemin expression and neural alterations in chronic pancreatitis was examined using the Spearman Rho test with the correlation coefficient and the corresponding p value. Two sided p values were always computed and an effect was considered statistically significant at a p value ⩽0.05.
Results
Expression of artemin and GFRα3 in different aetiologies of chronic pancreatitis
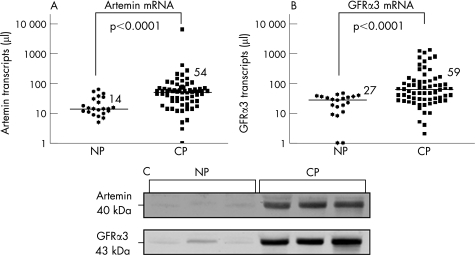
Quantitative RT‐PCR analysis was performed to investigate artemin mRNA expression in normal pancreatic and chronic pancreatitis tissues. In chronic pancreatitis, the artemin mRNA level of 54 (25–71) transcripts/μl (median; IQR) was significantly (p<0.0001) higher than that of normal pancreas, with 14 (11–33) transcripts/μl (fig 1A). Similar results were obtained for GFRα3, with significantly increased mRNA transcripts/μl in chronic pancreatitis (59; 28–118) compared with normal pancreas (27; 14–36) (p<0.0001) (fig 1B). Western blot analysis revealed a striking increase for artemin and GFRα3 in chronic pancreatitis compared with normal pancreas (fig 1C). Artemin mRNA levels were significantly (p<0.01) higher in alcoholic and idiopathic chronic pancreatitis as well as in autoimmune pancreatitis compared with biliary chronic pancreatitis (table 2). The level of GFRα3 expression was not associated with the aetiology of chronic pancreatitis (table 2).
Figure 1 Artemin and glial derived neurotrophic factor family receptor (GFR)α3 expression in pancreatic tissues. Artemin (A) and GFRα3 (B) mRNA expression analysed by quantitative reverse transcription‐polymerase chain reaction (QRT‐PCR) in chronic pancreatitis (CP) (n = 66) and in normal pancreas (NP) (n = 22). Transcript numbers are presented on a logarithmic scale. The horizontal bars represent the median values. (C) Western blot analysis of artemin and GFRα3 mirrors the upregulation of artemin mRNA expression. Note the significant increase in both proteins in chronic pancreatitis compared with the normal pancreas.
Table 2 Relationship between the aetiology of chronic pancreatitis and mRNA expression of artemin and GFRα3.
| Normal pancreas (n = 22) | Alcoholic CP (n = 30) | Biliary CP (n = 8) | Idiopathic CP (n = 22) | AIP (n = 6) | p Value | |
|---|---|---|---|---|---|---|
| Artemin | 14 (11–33) | 58 (37–78) | 17.5 (8–28) | 50.5 (25–69) | 53.5 (44–55) | <0.01 |
| GFRα3 | 27 (14–36) | 65 (33–188) | 21 (2–40) | 64 (25–89) | 32 (18–46) | = 0.08 |
AIP, autoimmune pancreatitis; CP, chronic pancreatitis; GFRα3, glial derived neurotrophic factor family receptor α3.
Values are median (IQR) mRNA transcripts/μl.
Immunolocalisation of artemin and GFRα3 in chronic pancreatitis
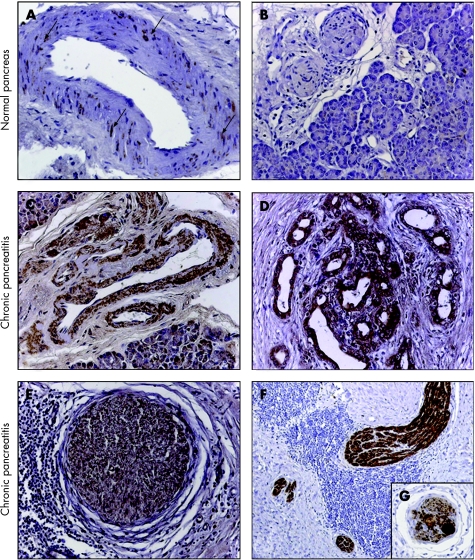
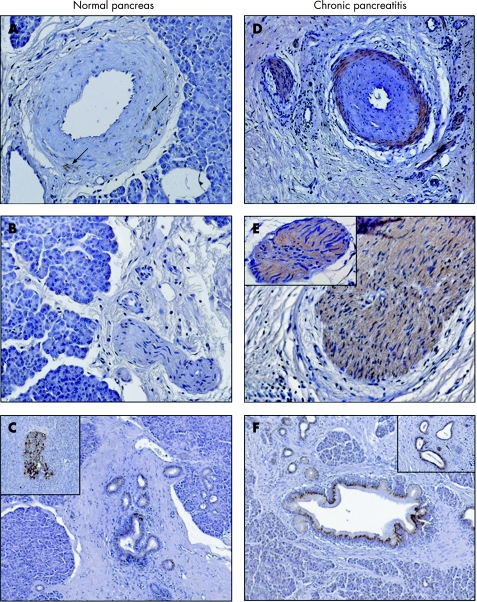
To determine the localisation of artemin and GFRα3 in chronic pancreatitis and in the normal pancreas, immunohistochemical analysis was performed. In the normal pancreas, weak artemin immunoreactivity was only detected in the smooth muscle cells of the arteries (fig 2A, B; table 3). GFRα3 immunoreactivity in the normal pancreas showed a similar pattern, with additional moderate and strong immunoreactivity in normal ducts and islets, respectively (fig 3A–C; table 3). In chronic pancreatitis, the immunoreactivity pattern of artemin changed dramatically. In addition to intense staining in the smooth muscle cells of arteries, strong immunoreactivity was also detected in tubular complexes and intrapancreatic nerves (fig 2C–F; table 3). Axons, and peri‐ and endoneurium were artemin negative whereas strong artemin immunoreactivity was found in the cytoplasm and nuclei of Schwann cells (fig 2E, F) and in intrapancreatic ganglia (fig 2G). Similar, but less prominent, changes were observed for GFRα3 in chronic pancreatitis (fig 3D–F; table 3). In nerves, moderate GFRα3 immunoreactivity was present in the cytoplasm but not in the nuclei of Schwann cells. Neurons of intrapancreatic ganglia, axons, and the peri‐ and endoneurium exhibited no GFRα3 immunoreactivity (fig 2E, 3E).
Figure 2 Immunohistochemical analysis of artemin in normal pancreas (A, B) and in chronic pancreatitis (D–G). In normal pancreatic tissue samples, immunostaining of artemin was only faintly present in the smooth muscle cells of arteries (see arrows in (A)). Acini, and intrapancreatic nerves and ducts did not reveal artemin immunoreactivity. In chronic pancreatitis, artemin showed increased immunoreactivity in the smooth muscle cells of arteries (C), in tubular complexes (D) and in the neural components (E–G). In nerves, artemin was present in the cytoplasm and nuclei of Schwann cells (E, F) and in intrapancreatic neural ganglia (G). Magnification 200× in all images except E; E 100×; chromogen: DAB.
Table 3 Semiquantitative analysis of artemin and GFRα3 immunoreactivity in normal pancreas and in chronic pancreatitis.
| Acini | Ductal cells | Tubular complexes | Ganglia | Nerves | Arteries | Inflammatory cells | Islets | Connective tissue | |
|---|---|---|---|---|---|---|---|---|---|
| NP | |||||||||
| Artemin | – | – | – | – | + | – | – | – | |
| GFRα3 | – | ++ | – | – | –/+ | – | +++ | – | |
| CP | |||||||||
| Artemin | –/+ | ++ | +++ | +++ | +++ | +++ | – | – | – |
| GFRα3 | + | ++ | ++ | – | ++ | ++ | –/+ | +++ | – |
–, absent; +, weak; ++, moderate; +++, strong immunoreactivity; CP, chronic pancreatitis; NP, normal pancreas; GFRα3, glial derived neurotrophic factor family receptor α3.
Figure 3 Immunohistochemical analysis of glial derived neurotrophic factor family receptor (GFR)α3 in normal pancreas (A–C) and in chronic pancreatitis (D–F). In normal pancreatic tissue samples, immunostaining of GFRα3 was minimally present in the smooth muscle cells of arteries (see arrows in (A)) and moderate to strong in ductal cells (C) and in islets (inset (C)). In chronic pancreatitis, GFRα3 showed moderate immunoreactivity in the smooth muscle cells of arteries (D), in nerve fibres, in the cytoplasm of Schwann cells (D, E) and in tubular complexes (F and inset F). Magnification 200× in images A, B and E; 100× in images C, D and F; chromogen: DAB.
Correlation of artemin and GFRα3 with inflammatory reactions in chronic pancreatitis
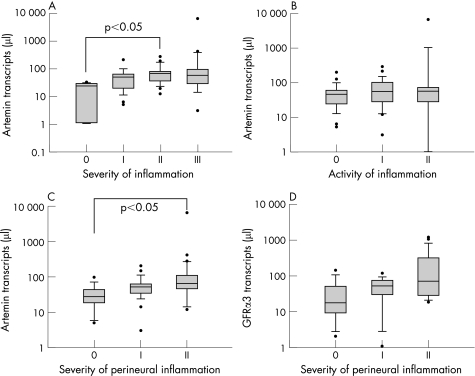
To investigate possible influences of acute (neutrophils) and chronic (macrophages, and B and T lymphocytes) inflammatory cell infiltrates in chronic pancreatitis on artemin expression, patients were analysed according to the described grading system. Expression of artemin was significantly higher in tissues that displayed increased severity of inflammation (scores I–III) than in tissues without inflammation (score 0) (p<0.03) (table 4, fig 4A). Moreover, expression of artemin mRNA showed a significant increase in group II compared with group 0 (p<0.05) (fig 4A). In contrast, there was no significant difference in GFRα3 expression with regard to the severity of inflammation (table 4). Similarly, there was no relationship between either artemin or GFRα3 mRNA expression and the activity of inflammation (table 4, fig 4B).
Table 4 Relationship of artemin and GFRα3 mRNA expression with different pathomorphological criteria in 66 patients with chronic pancreatitis.
| Score | Severity of inflammation | Activity of inflammation | Severity of perineural inflammation | Activity of perineural inflammation | Severity of fibrosis | Severity of pain |
|---|---|---|---|---|---|---|
| 0 | n = 6 | n = 23 | n = 14 | n = 43 | n = 10 | n = 18 |
| I | n = 23 | n = 28 | n = 24 | n = 14 | n = 29 | n = 8 |
| II | n = 21 | n = 15 | n = 28 | n = 9 | n = 27 | n = 40 |
| III | n = 16 | – | – | – | – | – |
| Artemin KW value | 9.2 | 1.1 | 8.5 | 0.8 | 6.3 | 11.2 |
| p Value | 0.03* | 0.57 | 0.04* | 0.67 | 0.04* | 0.003* |
| GFRα3 KW value | 3.7 | 3.3 | 3.2 | 7.6 | 1.4 | 2.1 |
| p Value | 0.30 | 0.19 | 0.37 | 0.02* | 0.50 | 0.35 |
GFRα3, glial derived neurotrophic factor family receptor α3; KW, Kruskal–Wallis.
Figure 4 Correlation of artemin with pathomorphological inflammatory changes in chronic pancreatitis (n = 66). Transcript numbers are presented on a logarithmic scale. The horizontal bars represent the median values and the error bars IQR. (A) Expression of artemin mRNA transcripts was positively related to the severity of inflammation in chronic pancreatitis (p<0.03). (B) The activity of inflammation did not correlate with artemin expression. (C) Artemin mRNA expression was positively related to the severity of perineural inflammation (p<0.04). (D) Glial derived neurotrophic factor family receptor (GFR)α3 mRNA expression showed a tendency to increase with the severity of perineural inflammation but failed to reach statistical significance.
The next step was to study possible influences of the perineural inflammatory cell infiltration on artemin expression. The severity of perineural inflammation was positively related to artemin mRNA levels (p<0.05) (table 4, fig 4C), and furthermore revealed a significant increase among patients in groups 0 and II (p<0.05) (fig 4C). GFRα3 mRNA expression showed a tendency to increase with the severity of perineural inflammation but did not reach statistical significance (table 4, fig 4D). No relationship was observed between artemin mRNA expression and the activity of perineural inflammation (table 4). However, GFRα3 mRNA expression revealed a negative relationship with the activity of perineural inflammation (p<0.02) (table 4).
Correlation of artemin and GFRα3 with neural alterations in chronic pancreatitis
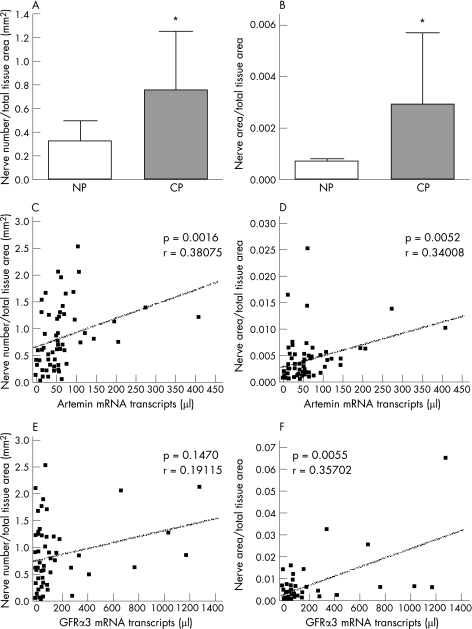
To study whether artemin and GFRα3 overexpression are associated with neural alterations in chronic pancreatitis, NHY and neural density were related to artemin and GFRα3 mRNA expression. Quantitative analysis of the neural alterations revealed a major increase in the number (neural density) and total area of nerves (NHY) in chronic pancreatitis (p<0.001) compared with the normal pancreas (fig 5A, B). The increase in neural density (p<0.002) and enhancement of NHY (p<0.006) were both positively correlated with the continuous rise of artemin mRNA in chronic pancreatitis (fig 5C, D). GFRα3 was also positively correlated with NHY (p<0.005) but failed to reach statistical significance when correlated with neural density (p = 0.147) (fig 5E, F).
Figure 5 Correlation of artemin with neural alterations in chronic pancreatitis (n = 66). After PGP9.5 staining, the whole chronic pancreatitis tissues were scanned and the marked nerves were counted using the software for Zeiss KS300, Zeiss AxioCam HR. Nerves in chronic pancreatitis increased (A) in total nerve number (neural sprouting) and (B) in the total area of nerves (neural hypertrophy) (*p<0.001) compared with the normal pancreas (NP). (C) Spearman Rho correlation revealed a positive correlation between artemin mRNA expression and neural density (p<0.002) as well as neural hypertrophy (p<0.006) (D) in chronic pancreatitis (CP). Glial derived neurotrophic factor family receptor (GFR)α3 was also positively correlated with neural hypertrophy (p<0.005) (E) but did not show a significant correlation with neural density (F).
Influence of artemin on pain in chronic pancreatitis
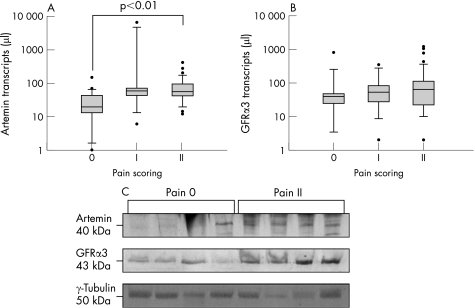
To evaluate a possible relationship between artemin and pain in chronic pancreatitis, artemin mRNA and protein levels were investigated in the groups pain 0, pain I and pain II. Artemin mRNA expression was positively correlated to the severity of pain in chronic pancreatitis (table 4) and was significantly (p<0.01) increased in the pain II group (56; 39–95 transcripts/μl) compared with the pain 0 group (19; 12–44 transcripts/μl) (fig 6A). In contrast, no relationship was observed between GFRα3 mRNA levels and pain (table 4, fig 6B). Densitometry analysis of artemin (p<0.03) and GFRα3 (p<0.03) protein levels revealed a significant increase in patients with moderate to severe pain (pain II) compared with patients without pain (pain 0) (fig 6C).
Figure 6 Relationship of artemin and glial derived neurotrophic factor family receptor (GFR)α3 to pain in chronic pancreatitis (n = 66). Transcript numbers are presented on a logarithmic scale. The horizontal bars represent the median values and the error bars the IQR. (A) Artemin mRNA expression was positively related to the severity of pain in chronic pancreatitis (p<0.001). (B) GFRα3 mRNA expression was not related to pain in chronic pancreatitis. (C) Western blot analysis identified a steady increase in artemin and GFRα3 in patients in the group with no pain (pain 0; n = 4) compared with the group with moderate to severe pain (pain II; n = 4).
Influence of stromal fibrosis on artemin expression, neural alterations and inflammation in chronic pancreatitis
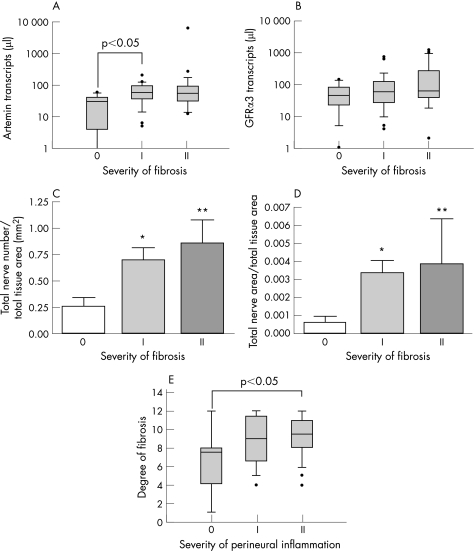
Increased artemin mRNA expression was positively correlated with the severity of fibrosis in chronic pancreatitis. As such, artemin expression showed a significant increase in the fibrosis group I (57; 35–86 transcripts/μl) compared with group 0 (25; 10–38 transcripts/μl) (p<0.05) (table 4, fig 7A). On the other hand, GFRα3 expression did not show any significant relationship (table 4, fig 7B). To explore whether the tissue damage and changes in the extracellular matrix influence or even trigger neural alterations in chronic pancreatitis, neural density and hypertrophy were correlated with the severity of fibrosis. Interestingly, increased neural density (p<0.01; Kruskal–Wallis 9.3) and NHY (p<0.005; Kruskal–Wallis 10.9) were both positively correlated with the severity of fibrosis in chronic pancreatitis (fig 7C, D). Dunn's multiple comparison test revealed a significant increase in neural density and NHY in fibrosis group I (p<0.05) which further increased in group II (p<0.01) compared with group 0 (fig 7C, D). Perineural invasion by inflammatory cells was also positively correlated with the degree of fibrosis (p<0.03; Kruskal–Wallis 7.1) and reached statistical significance between groups 0 and II (p<0.05) (fig 7E).
Figure 7 Influence of severity of fibrosis on artemin expression, neural alterations and perineural inflammation in chronic pancreatitis (n = 66). Transcript numbers of artemin and glial derived neurotrophic factor family receptor (GFR)α3 are presented on a logarithmic scale. The horizontal bars represent the median values and the error bars IQR. (A) Artemin mRNA was positively correlated with the severity of fibrosis in chronic pancreatitis (p<0.04) whereas GFRα3 expression (B) did not show any relationship. (C) Increased neural sprouting (total nerve number) (p<0.01) and (D) enhanced neural hypertrophy (total nerve area) (p<0.005) were both positively related to the severity of fibrosis in chronic pancreatitis. Within the groups, significant increases in neural density and hypertrophy were detected in the severity of fibrosis group I (*p<0.05), with even further increases in group II (**p<0.01) compared with group 0. (E) The severity of perineural inflammation was positively related to the degree of fibrosis (p<0.03; Kruskal–Wallis value 7.1) in chronic pancreatitis.
Immunoreactivity of artemin and GFRα3 in hPSCs and the effects of fibrogenic growth factors on artemin mRNA expression
To identify novel sources of artemin in chronic pancreatitis, we studied the influence of hPSCs. HPSCs demonstrated low levels of artemin mRNA expression (6 transcripts/μl). Incubation of hPSCs with TGFβ1 induced a 100% increase in artemin mRNA expression whereas FGF‐I, FGF‐II, PDGF‐AA and PDGF‐BB did not alter artemin mRNA levels. However, artemin and GFRα3 proteins were below the detection limit of immunofluorescence and western blot analyses.
Discussion
The neurotrophic factor artemin was recently found to exert antinociceptive and neuroprotective effects in an experimental model of neuropathic pain.25 These findings prompted us to investigate whether artemin might also be involved in the neuroimmune interactions in chronic pancreatitis, and whether it could be a novel candidate for possible therapeutic applications for pain management in patients with chronic pancreatitis.
Our study links artemin to pathophysiological changes associated with sustained abdominal pain in patients with chronic pancreatitis. For the first time, we have demonstrated that artemin and its coreceptor GFRα3 are overexpressed in chronic pancreatitis. Acinar cell destruction and replacement of the lost parenchyma by dense fibrotic tissue in chronic pancreatitis is accompanied by inflammatory cell infiltration, neural and vascular hypertrophy, and intrapancreatic neural alterations.1,2,3 Our analysis revealed that artemin expression is directly related to histopathological changes such as inflammatory cell infiltration, pancreatitis associated neuritis, neural morphological changes and fibrosis in chronic pancreatitis. Furthermore, artemin overexpression was significantly associated with pain in patients with chronic pancreatitis.
The destruction of the pancreatic parenchyma in chronic pancreatitis is accompanied by a prominent infiltration of different subsets of inflammatory cells.1,2,3,35,36 These cells can easily infiltrate the nerve via the disrupted perineurium and cause a local pancreatitis associated neuritis. This phenomenon is directly correlated to pain in patients with chronic pancreatitis.8,37 As artemin positive cells are not located in the perineurium but rather within the nerve fascicles, loss of the perineurium integrity seems to precede artemin upregulation in chronic pancreatitis. Artemin is an important regulator of neural proliferation and regeneration under physiological conditions.16,19,23 Moreover, under pathological circumstances, GFRα3 is upregulated in distal nerve segment filaments (ie, sciatic transsection) in order to regenerate the neural components.38,39 Thus we hypothesise that following initial damage to intrapancreatic nerves by infiltrating immune cells, intrapancreatic ganglia and/or Schwann cells locally produce excessive artemin in an effort to initiate and maintain neural regeneration. Furthermore, continuing damage and irritation of intrapancreatic nerves by immune cells may lead to activation of neurons in the dorsal root ganglia which could contribute to extrapancreatic artemin production. As known for the other GDNF family members,40 it is possible that artemin is then transported to the pancreas via retrograde axonal transport in sensory afferents, in order to restore intrapancreatic neural integrity.
As artemin increases neuronal density and neurite outgrowth of sympathetic neurons,16,19,24 its strong presence in intrapancreatic nerves and ganglia, together with that of its coreceptor GFRα3, suggests that it may be an important mediator of neuronal proliferation in chronic pancreatitis. Therefore, we believe that increased artemin and GFRα3 mRNA expression may lead to enhanced neural density and neural hypertrophy in chronic pancreatitis. These findings are in agreement with those of Bolon et al, who reported that exogenous artemin administration leads to neural dysplasia with enlargement of sympathetic and parasympathetic ganglia in mice.41
Artemin seems to be correlated with pancreatitis associated neuritis and neural alterations in chronic pancreatitis, and therefore we analysed whether it is also associated with pain. Analysis of artemin mRNA expression levels correlated positively with the severity of pain in patients with chronic pancreatitis. Although artemin demonstrated antinociceptive effects in an animal model of spinal nerve ligation,25 it is probable that endogenous levels of artemin in chronic pancreatitis are not sufficient to block pain. Indeed, even in the rat model of spinal nerve injury, repeated injections of several milligrams of recombinant artemin were required to achieve a therapeutic effect.25 Another explanation could be that artemin is able to reverse pain and neurochemical changes induced by mechanical injury but is unable to attenuate nociception at the sites of chronic inflammation, such as chronic pancreatitis with longstanding neurogenic inflammation and inflammatory neuropathy.
Artemin expression was also associated with the severity of fibrosis in chronic pancreatitis. In patients with chronic pancreatitis, artemin expression increased in parallel with the extent of parenchymal destruction and stromal fibrosis. As far as we know, a profibrogenic feature of artemin has not yet been identified. Therefore, we investigated the presence of a possible link between fibrogenesis and artemin overexpression. As there is a dramatic increase in the number of stellate cells in chronic pancreatitis,42,43 we analysed their responses to fibrogenic growth factors in terms of artemin production. Although both FGFs and PDGFs failed to stimulate artemin expression, TGFβ1 doubled its production in hPSCs. Therefore, hPSCs may also contribute to increased neural density and hypertrophy in chronic pancreatitis, as artemin acts as a neural guidance molecule that induces enlargement, migration and axonal projection of sympathetic neurons.23,41 Such a link seems possible, as TGFβ1 is a pluripotent growth factor and cytokine which regulates fibrogenesis in chronic pancreatitis,44 with immune cells and hPSCs being the most prominent cellular sources.45 The perineural sheaths of the enlarged intrapancreatic nerves in chronic pancreatitis are altered and damaged in such a way that they no longer provide a protective barrier between the surrounding connective tissue and the internal neural components.5 Under such circumstances, cytokines such as TGFβ, released by inflammatory cells,46 might enter the internal neural components and stimulate Schwann cells, intrapancreatic neural ganglia and endoneural fibroblasts to induce artemin expression. Recent observations support our hypothesis that the GDNF family of ligands require TGFβ as a cofactor to exert their full neurotrophic potential in vivo and in vitro.47,48
In this study, we discovered for the first time that the severity of fibrosis in chronic pancreatitis is directly correlated with the severity of perineural inflammation and increased neural sprouting and hypertrophy. These results provide new insights into the neuroimmune interactions in chronic pancreatitis, and demonstrate that the extracellular matrix is actively involved in intrapancreatic neural alterations and consequently in pain generation. These novel observations require further investigation.
In summary, upregulated artemin and GFRα3 seem to be associated with neural alterations and pain in chronic pancreatitis. However, endogenous artemin does not seem to lead to antinociception in patients with chronic pancreatitis. As TGFβ1 stimulates artemin expression in hPSCs, the degree of pancreatic damage may influence neural alterations, in part via artemin.
The authors are grateful to Professor K‐H Schäfer for critical review of the manuscript and for technical support. The authors thank Ms Kathrin Schneider for her superior technical assistance. The authors are also very thankful to Jeannie Wurz for the native English correction.
Abbreviations
FCS - fetal calf serum
FGF - fibroblast growth factor
GDNF - glial derived neurotrophic factor
GFR - GDNF family receptor
hPSC - human pancreatic stellate cell
NHY - neural hypertrophy
PDGF - platelet derived growth factor
QRT‐PCR - quantitative reverse transcription‐polymerase chain reaction
TGF - transforming growth factor
Footnotes
Competing interests: None declared.
References
- 1.Adler G, Schmid R M. Chronic pancreatitis: still puzzling? Gastroenterology 19971121762–1765. [DOI] [PubMed] [Google Scholar]
- 2.Sarles H, Bernard J P, Johnson C. Pathogenesis and epidemiology of chronic pancreatitis. Annu Rev Med 198940453–468. [DOI] [PubMed] [Google Scholar]
- 3.Dimagno E P. A short, eclectic history of exocrine pancreatic insufficiency and chronic pancreatitis. Gastroenterology 19931041255–1262. [DOI] [PubMed] [Google Scholar]
- 4.Di Sebastiano P, di Mola F F, Buchler MW et a l. Pathogenesis of pain in chronic pancreatitis. Dig Dis 200422267–272. [DOI] [PubMed] [Google Scholar]
- 5.Bockman D E, Buchler M, Malfertheiner P.et al Analysis of nerves in chronic pancreatitis. Gastroenterology 1988941459–1469. [DOI] [PubMed] [Google Scholar]
- 6.Friess H, Shrikhande S, Shrikhande M.et al Neural alterations in surgical stage chronic pancreatitis are independent of the underlying aetiology. Gut 200250682–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keith R G, Keshavjee S H, Kerenyi N R. Neuropathology of chronic pancreatitis in humans. Can J Surg 198528207–211. [PubMed] [Google Scholar]
- 8.Di Sebastiano P, Fink T, Weihe E.et al Immune cell infiltration and growth‐associated protein 43 expression correlate with pain in chronic pancreatitis. Gastroenterology 19971121648–1655. [DOI] [PubMed] [Google Scholar]
- 9.Bockman D E. Morphology of pancreatic nerves in health and disease. In: Buchler MW, Friess H, Uhl W, Malfertheiner P, eds. Chronic pancreatitis. Novel concepts in biology and therapy Oxford: Blackwell Publishing, 2002163–169.
- 10.Fink T, Di Sebastiano P, Buchler M.et al Growth‐associated protein‐43 and protein gene‐product 9. 5 innervation in human pancreas: changes in chronic pancreatitis, Neuroscience 199463249–266. [DOI] [PubMed] [Google Scholar]
- 11.Friess H, Zhu Z W, di Mola F F.et al Nerve growth factor and its high‐affinity receptor in chronic pancreatitis. Ann Surg 1999230615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Z W, Friess H, Wang L.et al Nerve growth factor exerts differential effects on the growth of human pancreatic cancer cells. Clin Cancer Res 20017105–112. [PubMed] [Google Scholar]
- 13.Kotzbauer P T, Lampe P A, Heuckeroth R O.et al Neurturin, a relative of glial‐cell‐line‐derived neurotrophic factor. Nature 1996384467–470. [DOI] [PubMed] [Google Scholar]
- 14.Milbrandt J, de Sauvage F J, Fahrner T J.et al Persephin, a novel neurotrophic factor related to GDNF and neurturin. Neuron 199820245–253. [DOI] [PubMed] [Google Scholar]
- 15.Baloh R H, Tansey M G, Johnson E M., Jret al. Functional mapping of receptor specificity domains of glial cell line‐derived neurotrophic factor (GDNF) family ligands and production of GFRalpha1 RET‐specific agonists. J Biol Chem 20002753412–3420. [DOI] [PubMed] [Google Scholar]
- 16.Baloh R H, Tansey M G, Lampe P A.et al Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3‐RET receptor complex. Neuron 1998211291–1302. [DOI] [PubMed] [Google Scholar]
- 17.Saarma M G. GDNF‐a stranger in the FGF‐beta superfamily? Eur J Biochem 20002676968–6971. [DOI] [PubMed] [Google Scholar]
- 18.Baudet C, Mikaels A, Westphal H.et al Positive and negative interactions of GDNF, NTN and ART in developing sensory neuron subpopulations, and their collaboration with neurotrophins. Development 20001274335–4344. [DOI] [PubMed] [Google Scholar]
- 19.Enomoto H, Crawford P A, Gorodinsky A.et al RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development 20011283963–3974. [DOI] [PubMed] [Google Scholar]
- 20.Baloh R H, Gorodinsky A, Golden J P.et al GFRalpha3 is an orphan member of the GDNF/neurturin/persephin receptor family. Proc Natl Acad Sci U S A 1998955801–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Worby C A, Vega Q C, Chao H H.et al Identification and characterization of GFRalpha‐3, a novel co‐receptor belonging to the glial cell line‐derived neurotrophic receptor family. J Biol Chem 19982733502–3508. [DOI] [PubMed] [Google Scholar]
- 22.Coulpier M, Anders J, Ibanez C F. Coordinated activation of autophosphorylation sites in the RET receptor tyrosine kinase: importance of tyrosine 1062 for GDNF mediated neuronal differentiation and survival. J Biol Chem 20022771991–1999. [DOI] [PubMed] [Google Scholar]
- 23.Honma Y, Araki T, Gianino S.et al Artemin is a vascular‐derived neurotropic factor for developing sympathetic neurons. Neuron 200235267–282. [DOI] [PubMed] [Google Scholar]
- 24.Andres R, Forgie A, Wyatt S.et al Multiple effects of artemin on sympathetic neurone generation, survival and growth. Development 20011283685–3695. [DOI] [PubMed] [Google Scholar]
- 25.Gardell L R, Wang R, Ehrenfels C.et al Multiple actions of systemic artemin in experimental neuropathy. Nat Med 200391383–1389. [DOI] [PubMed] [Google Scholar]
- 26.Guo J, Kleeff J, Li J.et al Expression and functional significance of CDC25B in human pancreatic ductal adenocarcinoma. Oncogene 20042371–81. [DOI] [PubMed] [Google Scholar]
- 27.Koninger J, Balaz P, Wagner M.et al Phosphatidylserine receptor in chronic pancreatitis: evidence for a macrophage independent role. Ann Surg 2005241144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ceyhan G O, Giese N A, Erkan M.et al The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg 2006244274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ammann R W, Heitz P U, Kloppel G. Course of alcoholic chronic pancreatitis: a prospective clinicomorphological long‐term study. Gastroenterology 1996111224–231. [DOI] [PubMed] [Google Scholar]
- 30.Shrikhande S V, Friess H, di Mola F F.et al NK‐1 receptor gene expression is related to pain in chronic pancreatitis. Pain 200191209–217. [DOI] [PubMed] [Google Scholar]
- 31.Friess H, Shrikhande S, Shrikhande M.et al Neural alterations in surgical stage chronic pancreatitis are independent of the underlying aetiology. Gut 200250682–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Sebastiano P, Fink T, Weihe E.et al Changes of protein gene product 9.5 (PGP 9.5) immunoreactive nerves in inflamed appendix. Dig Dis Sci 199540366–372. [DOI] [PubMed] [Google Scholar]
- 33.Bachem M G, Schneider E, Gross H.et al Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998115421–432. [DOI] [PubMed] [Google Scholar]
- 34.Bachem M G, Schneider E, Gross H.et al Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998115421–432. [DOI] [PubMed] [Google Scholar]
- 35.Malfertheiner P, Buchler M, Stanescu A.et al Exocrine pancreatic function in correlation to ductal and parenchymal morphology in chronic pancreatitis. Hepatogastroenterology 198633110–114. [PubMed] [Google Scholar]
- 36.Sarner M, Cotton P B. Definitions of acute and chronic pancreatitis. Clin Gastroenterol 198413865–870. [PubMed] [Google Scholar]
- 37.Di Sebastiano P, di Mola F F, Bockman D E.et al Chronic pancreatitis: the perspective of pain generation by neuroimmune interaction. Gut 200352907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett D L, Boucher T J, Armanini M P.et al The glial cell line‐derived neurotrophic factor family receptor components are differentially regulated within sensory neurons after nerve injury. J Neurosci 200020427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orozco O E, Walus L, Sah D W.et al GFRalpha3 is expressed predominantly in nociceptive sensory neurons. Eur J Neurosci 2001132177–2182. [DOI] [PubMed] [Google Scholar]
- 40.Leitner M L, Molliver D C, Osborne P A.et al Analysis of the retrograde transport of glial cell line‐derived neurotrophic factor (GDNF), neurturin, and persephin suggests that in vivo signaling for the GDNF family is GFRalpha coreceptor‐specific. J Neurosci 1999199322–9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolon B, Jing S, Asuncion F.et al The candidate neuroprotective agent artemin induces autonomic neural dysplasia without preventing peripheral nerve dysfunction. Toxicol Pathol 200432275–294. [DOI] [PubMed] [Google Scholar]
- 42.Hasel C, Durr S, Rau B.et al In chronic pancreatitis, widespread emergence of TRAIL receptors in epithelia coincides with neoexpression of TRAIL by pancreatic stellate cells of early fibrotic areas. Lab Invest 200383825–836. [DOI] [PubMed] [Google Scholar]
- 43.Kloppel G, Detlefsen S, Feyerabend B. Fibrosis of the pancreas: the initial tissue damage and the resulting pattern. Virchows Arch 20044451–8. [DOI] [PubMed] [Google Scholar]
- 44.Kayed H, Muller M, Kleeff J.et al Molecular alterations in chronic pancreatitis. J Hepatobiliary Pancreat Surg 20029653–658. [DOI] [PubMed] [Google Scholar]
- 45.van Laethem J L, Deviere J, Resibois A.et al Localization of transforming growth factor beta 1 and its latent binding protein in human chronic pancreatitis. Gastroenterology 19951081873–1881. [DOI] [PubMed] [Google Scholar]
- 46.Stevens T, Conwell D L, Zuccaro G. Pathogenesis of chronic pancreatitis: an evidence‐based review of past theories and recent developments. Am J Gastroenterol 2004992256–2270. [DOI] [PubMed] [Google Scholar]
- 47.Schober A, Hertel R, Arumae U.et al Glial cell line‐derived neurotrophic factor rescues target‐deprived sympathetic spinal cord neurons but requires transforming growth factor‐beta as cofactor in vivo. J Neurosci 1999192008–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krieglstein K, Strelau J, Schober A.et al TGF‐beta and the regulation of neuron survival and death. J Physiol Paris 20029625–30. [DOI] [PubMed] [Google Scholar]