Abstract
Objective
Although the epidemiology of microscopic colitis has been described in Europe, no such data exist from North America. We studied the incidence, prevalence and temporal trends of microscopic colitis in a geographically defined US population.
Design and setting
In this population based cohort study, residents of Olmsted County, Minnesota, with a new diagnosis of microscopic colitis, and all who had colon biopsies for evaluation of diarrhoea, between 1 January 1985 and 31 December 2001 were identified. Biopsies were reviewed for confirmation (cases) and to identify missed cases (diarrhoea biopsies).
Main outcome measures
Incidence rates, age and sex adjusted to the 2000 US white population. Poisson regression assessed the association of calendar period, age and sex with incidence.
Results
We identified 130 incident cases for an overall rate of 8.6 cases per 100 000 person‐years. There was a significant secular trend, with incidence increasing from 1.1 per 100 000 early in the study to 19.6 per 100 000 by the end (p<0.001). Rates increased with age (p<0.001). By subtype, the incidence was 3.1 per 100 000 for collagenous colitis and 5.5 per 100 000 for lymphocytic colitis. Collagenous colitis was associated with female sex (p<0.001) but lymphocytic colitis was not. Prevalence (per 100 000 persons) on 31 December 2001 was 103.0 (39.3 for collagenous colitis and 63.7 for lymphocytic colitis).
Conclusions
The incidence of microscopic colitis has increased significantly over time, and by the end of the study, the incidence and prevalence were significantly higher than reported previously. Microscopic colitis is associated with older age, and collagenous colitis is associated with female sex.
The term “microscopic colitis” was introduced in 1980 to describe patients with chronic watery diarrhoea who had lymphocytic inflammation in biopsies from grossly normal colons.1 Collagenous colitis is a closely related condition with similar clinical and histological features but in addition the subepithelial collagen band is thickened.2 As the colon in collagenous colitis is also grossly normal, it is also considered a form of microscopic colitis. Therefore, the current approach to nomenclature is to use the term “microscopic colitis” as an umbrella term with two major subsets: collagenous colitis, with a thickened subepithelial collagen band, and lymphocytic colitis, with no subepithelial collagen thickening.3
Several European studies have investigated the epidemiology of these disorders. The annual incidence of collagenous colitis has been reported as 0.6–5.2 cases per 100 000 with a prevalence of 10–15.7 per 100 000.4,5,6,7,8 The epidemiology of lymphocytic colitis has been less well studied but an annual incidence of 3.1–4.4 per 100 000 and a prevalence of 14.2 per 100 000 have been reported.6,7,8,9 In some of these studies, the incidence of collagenous or lymphocytic colitis was shown to increase over time, but this was not seen in other studies,5,6,7,8 and these trends were not analysed statistically in some reports.5,7 The incidence and prevalence of these conditions in the US are unknown, and temporal trends in the incidence of these disorders in the US have not been studied. Anecdotally, many clinicians have appreciated an apparent increase in cases; however, it is not known whether this represents referral centre bias, increased diagnostic awareness of these conditions or a true increase in incidence.
Population based descriptions of incidence and prevalence are important for estimating disease burden in the community which can inform decisions about resource allocation, not only for health care resources, but for research resources as well.
Thus we investigated the incidence, prevalence and time trends of microscopic colitis and its subtypes in a descriptive study in Olmsted County, Minnesota, where population based investigation of chronic disease is possible through the medical records linkage system of the Rochester Epidemiology Project.
Methods
Study setting
Olmsted County is situated in southeastern Minnesota and had approximately 124 000 residents at the time of the 2000 US Census. The county has an urban centre, Rochester, which had a population of approximately 86 000 people in 2000. The remainder of the county is predominantly rural. In 2000, 89% of the population was non‐Hispanic white. Although 25% of the county residents are employed in health care services (compared with 8% nationwide), and the level of education is higher (30% have completed college compared with 21% nationwide), the residents of Olmsted County are otherwise socioeconomically similar to the US white population.10
Rochester Epidemiology Project
The Rochester Epidemiology Project is a unique data resource that exploits the fact that virtually all health care for the residents of Olmsted County is provided by two organisations: the Mayo Medical Center, consisting of the Mayo Clinic and its affiliated hospitals (Rochester Methodist and Saint Mary's); and Olmsted Medical Center, consisting of another multispecialty clinic and its affiliated hospital (Olmsted Community Hospital). Diagnoses generated from all outpatient care, emergency room visits, hospitalisations, nursing home visits, surgical procedures, autopsy examinations and death certificates for all Olmsted County residents are recorded in a central diagnostic index.10 In any 3 year period, over 90% of county residents are examined at one of the two health care systems.10 Thus it is possible to identify virtually all diagnosed cases of a given disease for which patients sought medical attention. The data resources of the Rochester Epidemiology Project have supported over 1600 studies of acute and chronic diseases to date.
Ascertainment of microscopic colitis
Following approval by the Institutional Review Boards of the Mayo Foundation and Olmsted Medical Center, all county residents diagnosed with microscopic colitis (collagenous colitis or lymphocytic colitis) from 1 January 1985 to 31 December 2001 were identified by one of two mechanisms. Potential cases were identified through the diagnostic index, using ICD‐9 code 558.9 and H‐ICDA‐8 codes 561 and 563.9 for the period 1994–2001, and a Mayo specific code for “non‐infectious colitis” for the period 1985–1993. Second, cases were identified through the Department of Pathology database using a text word search. The inpatient and outpatient medical records of candidate cases were then reviewed. Cases with a diagnosis of Crohn's disease or ulcerative colitis, those with an alternative explanation for diarrhoea at the time of the index biopsy and those without diarrhoea were excluded. Patients who resided outside of Olmsted County at the time of initial diagnosis of microscopic colitis were also excluded from incidence calculations, but were included in prevalence calculations if they resided in the county of prevalence data.
All biopsy specimens from remaining subjects were reviewed by an expert gastrointestinal pathologist (TCS) to confirm the diagnosis according to standard criteria.3 Specifically, patients with collagenous colitis had preserved crypt architecture, increased intraepithelial lymphocytes (⩾20 lymphocytes per 100 epithelial cells) with a mixed lamina propria inflammatory infiltrate and a subepithelial collagen band at least 10 μm thick, while patients with lymphocytic colitis had similar inflammatory changes without a thickened collagen band. In addition, all colon biopsies obtained for evaluation of Olmsted County residents with diarrhoea during the study period were reviewed by the pathologist. These cases were identified through billing records, crossing the procedure codes for colon biopsies (which included colonoscopy and sigmoidoscopy biopsies) with the code for “non‐specific diarrhoea”. Only subjects with non‐bloody diarrhoea and without an identified cause of diarrhoea were included.
Statistical analysis
For the purpose of calculating incidence rates, it was assumed that the entire county population was at risk. Confirmed cases of microscopic colitis were included in the numerator, and denominator age and sex specific person‐years were estimated from decennial census data with interpolation between census years.11 Standard errors and 95% confidence intervals (95% CI) were calculated for the incidence rates assuming that they followed a Poisson distribution.12 Prevalence was calculated using all patients with a diagnosis of microscopic colitis residing in Olmsted County on 31 December 2001 as the numerator and the estimated county population on this date as the denominator. Prevalent cases residing in Olmsted County, but who were diagnosed before becoming County residents (ie, non‐incident prevalent cases), were included. Incidence and prevalence rates were directly age or age and sex adjusted to the population structure of white persons in the US in 2000.
Trends in incidence rates over the period of the study (categorised as 1985–89, 1990–93, 1994–97 and 1998–2001), as well as potential associations with age (categorised as 0–39, 40–49, 50–59, 60–69, 70–79 and 80+ years) and sex, were evaluated using Poisson regression analysis.13 Both age and calendar period were considered continuous predictors in this analysis. Prevalence of the subtypes of microscopic colitis were analysed with a two sided, one sample test to determine if the proportions were significantly different (ie, compared to equal distribution of the two subtypes, or 50% each). Survival from date of diagnosis was calculated using the Kaplan–Meier survival method,14 and was compared with the expected survival for Minnesota whites, 1950–2000, using the log rank test.15
These analyses were repeated separately for lymphocytic colitis and collagenous colitis. All analyses were carried out using SAS (SAS Institute Inc., Cary, North Carolina, USA).
Results
The initial query of the diagnostic index and pathology database yielded 243 unique individuals, of whom 109 were excluded for not having diarrhoea or having an explanation other than microscopic colitis for their diarrhoea. The remaining 134 cases were reviewed histologically; four cases were excluded for not meeting the histological criteria for diagnosis and three were excluded for living outside the county at the time of diagnosis. In addition, 798 patients in whom colon biopsies were obtained from a normal appearing colon for evaluation of diarrhoea were reviewed, and three additional cases of microscopic colitis were identified. Among the remaining 795 cases, 700 were histologically unremarkable and 95 showed focal active colitis, presumably representing either infection, medication injury or bowel preparation effect.
Therefore, 130 cases with a new diagnosis of microscopic colitis were included in this study, out of the 929 county residents who had colon biopsies to evaluate diarrhoea that was not otherwise explained (14%). The median age at diagnosis was 68 years (range 24–94), and 91 (70%) were female. The overall age and sex adjusted incidence was 8.6 cases per 100 000 person‐years (95% CI 7.1 to 10.0). Fifty of the 130 patients were tested for coeliac sprue (45 with small bowel biopsies, with or without serology, and five with serology only) and five (two with collagenous colitis and three with lymphocytic colitis) were positive (10% of those tested, 3.8% of the entire cohort).
Results were also examined by subtype of microscopic colitis. There were 46 incident cases of collagenous colitis with a median age at diagnosis of 70 years (range 35–94); 87% were females. The overall age and sex adjusted annual incidence for this subset was 3.1 per 100 000 (95% CI 2.2 to 4.0). For lymphocytic colitis, there were 84 incident cases, with a median age at diagnosis of 68 years (range 24–90); 62% were female. The age and sex adjusted incidence for this subset was 5.5 per 100 000 (95% CI 4.3 to 6.6). The difference in incidence between the two subtypes was significant (p<0.01) but this resulted from an interaction with sex (p = 0.001) such that rates were significantly less for men with collagenous colitis compared with the other sex and type specific rates (table 1). Thus for collagenous colitis, but not lymphocytic colitis, incidence was associated with female sex (table 1).
Table 1 Sex specific incidence of microscopic colitis among Olmsted County, Minnesota, residents.
| Female | Male | p Value* | |
|---|---|---|---|
| Collagenous colitis | 4.8 (3.3 to 6.3) | 1.1 (0.2 to 2.0) | <0.001 |
| Lymphocytic colitis | 6.1 (4.4 to 7.7) | 5.0 (3.2 to 6.8) | 0.24 |
| All microscopic colitis | 11.0 (8.7 to 13.2) | 6.1 (4.1 to 8.1) | <0.001 |
Rates represent age adjusted incidence per 100 000 person‐years with 95% CI.
*p Values were obtained using Poisson regression models including age, sex and calendar period.
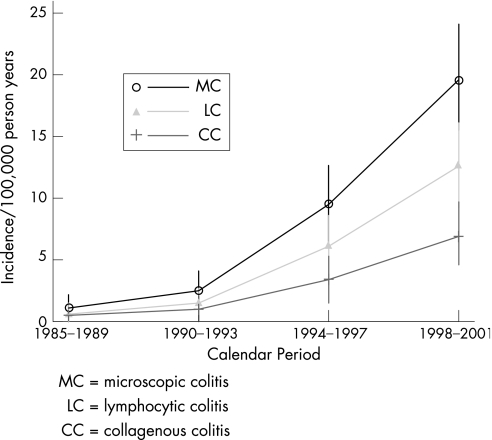
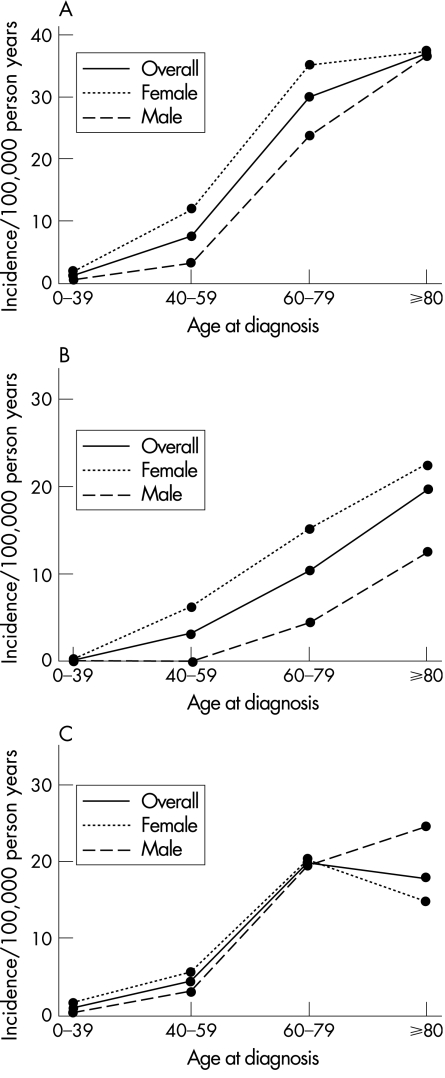
Poisson regression analysis showed that the incidence of microscopic colitis as a whole, as well as for both subtypes, increased significantly over the period of the study (p<0.001) (fig 1). The incidence rates, overall and for both subtypes, also increased significantly with advancing age (fig 2A–C), with a peak annual incidence of approximately 37 per 100 000 in patients ⩾80 years of age.
Figure 1 Age and sex adjusted incidence of microscopic colitis and subtypes over time among Olmsted County, Minnesota residents, 1985–2001.
Figure 2 (A) Age and sex specific incidence of microscopic colitis among Olmsted County, Minnesota residents. (B) Age and sex specific incidence of collagenous colitis among Olmsted County, Minnesota residents. (C) Age and sex specific incidence of lymphocytic colitis among Olmsted County, Minnesota residents.
Five year survival was 82.5% and was not significantly different than expected (84.3%, p = 0.8). On 31 December 2001, there were 111 county residents alive with microscopic colitis, including one prevalent case that had been diagnosed before moving to the county (and who therefore was not included in the incidence figures). The age and sex adjusted prevalence of microscopic colitis was 103 cases per 100 000 persons. There were 42 prevalent cases of collagenous colitis (39.3 per 100 000), and 69 prevalent cases of lymphocytic colitis (63.7 per 100 000). The difference in prevalence between the two subtypes was significant (p = 0.01). Current smokers accounted for 24% of the cohort (23% lymphocytic colitis, 27% collagenous colitis) while former smokers accounted for 28% (33% lymphocytic colitis, 22% collagenous colitis).
Discussion
This first population based epidemiological study of microscopic colitis in North America shows that microscopic colitis is relatively common, appears to be increasing with time, and is associated with older age.
Averaged over the entire study period, our incidence rates are similar to previous reports from Europe (table 2).
Table 2 Comparison of microscopic colitis incidence rates (per 100,000 person‐years) from the published literature.
| Country | Time period | Collagenous colitis | Lymphocytic colitis |
|---|---|---|---|
| France4 | 1987–1992 | 0.6 | |
| Sweden5 | 1984–1993 | 1.8 | |
| Sweden6 | 1993–1998 | 4.9 | 4.4 |
| Spain7 | 1993–1997 | 1.1 | 3.1 |
| Iceland8 | 1995–1999 | 5.2 | 4.0 |
| USA* | 1985–2001 | 3.1 | 5.5 |
| USA* | 1985–1997 | 1.6 | 2.7 |
| USA* | 1998–2001 | 7.1 | 12.6 |
*Current study.
However, incidence rates increased substantially over time and, by the end of the study, the incidence of collagenous colitis and lymphocytic colitis were both significantly greater than reported previously. Indeed, by the end of our study period, the incidence of microscopic colitis exceeded that of Crohn's disease and ulcerative colitis in Olmsted County.16,17 Previous studies have reported a significant secular increase in the incidence of collagenous colitis but not lymphocytic colitis8 or of lymphocytic colitis but not collagenous colitis.6 Other studies have reported either an increase in incidence5 or not,7 but in these reports, the differences were not analysed statistically.
The cause of the increase in incidence seen in our study is not clear based on our current understanding of the pathophysiology of microscopic colitis. Some cases of microscopic colitis appear to be medication related18 or postinfectious in nature.3 Therefore, exposure to certain medications or microorganisms may have increased over this study to account for the increase in incidence but more work will be necessary to clarify these issues. Some of the cases in this report had what appeared to be drug induced colitis, and we assessed drug use as a potential explanation for the rise in incidence seen in this study. Specifically, we analysed trends in use of medications that were thought to be temporally associated with the onset of diarrhoea in these patients. However, no significant associations with individual drugs over time were discovered.
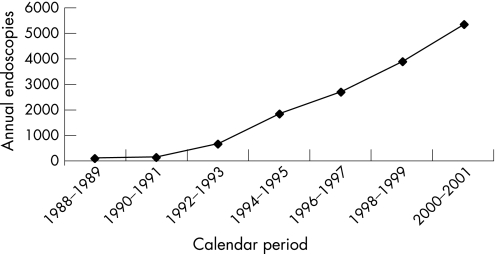
It is not clear if the trend seen in our study represents a true increase in disease incidence or whether it reflects instead diagnostic bias due to an increasing use of colonoscopy and sigmoidoscopy in county residents, which was evident during the study period (fig 3). Because of increasing awareness, gastroenterologists may have been more likely over the study period to obtain colon biopsies from normal mucosa in patients being evaluated for chronic diarrhoea.6 As all biopsies taken for evaluation of diarrhoea were reviewed, and only three additional cases were found, this increase in incidence does not appear to represent an increasing familiarity with these diagnoses among pathologists. In fact, the diagnosis of microscopic colitis appears to be reproducible with low interobserver variability, with only four of 134 previously diagnosed cases (3%) being excluded on histological grounds after review of biopsies, and only three of 798 biopsies (0.4%) previously read as not showing microscopic colitis being reclassified as showing microscopic colitis.
Figure 3 Number of colonoscopies and sigmoidoscopies performed on residents of Olmsted County, Minnesota, by calendar period.
In keeping with this increased incidence, the prevalence in our population was also significantly higher than previously reported. However, the higher prevalence seen in our study may be partly due to the fact that we counted any patient who was ever diagnosed with microscopic colitis in the numerator, regardless of whether or not they were symptomatic on the prevalence date. As some patients with microscopic colitis have self‐limited disease without having a recurrence, this prevalence figure may thus be an overestimation. In this study, microscopic colitis was diagnosed in approximately 14% of county residents who underwent biopsy of normal appearing colonic mucosa to evaluate diarrhoea, slightly higher than the 10% rate previously reported.6,7,8,19 Given this prevalence, biopsies should be obtained routinely in patients (particularly elderly patients) with chronic diarrhoea who are investigated with colonoscopy or sigmoidoscopy.
Similar to other studies, our results show a clear association between age and microscopic colitis. We also saw a significant association between female sex and collagenous colitis, but not lymphocytic colitis. In most epidemiological studies from Europe that assessed both subtypes, the association with female sex was also more significant in collagenous colitis than in lymphocytic colitis.6,7 However, in the study from Iceland, the association was high for both subtypes.8 The reason for the sex association in collagenous colitis but not in lymphocytic colitis in most studies is not known although hormonal influences on collagen metabolism have been postulated.3
We also found that lymphocytic colitis was more common than collagenous colitis. One European study showed that lymphocytic colitis was more common than collagenous colitis,7 another showed that collagenous colitis was more common8 and others showed similar incidence rates.6,8 The reason for these disparate findings is also not known but, if real, it may reflect different rates of exposure to agents that preferentially cause one subtype of microscopic colitis or the other. Another possible explanation is biopsy sampling error. In other words, as collagen deposition can be patchy throughout the colon, cases of collagenous colitis may have been misclassified in our study as lymphocytic colitis if biopsies happened to be taken from areas of the colon without significant collagen deposition.
In our cohort, 10% of patients who had specific testing were found to have coeliac sprue (or 3.8% of the entire cohort). These figures are similar to what has been reported previously3 and suggest that testing for this condition should be considered in patients with microscopic colitis, particularly those who have evidence of malabsorption (weight loss, steatorrhoea, unexplained metabolic bone disease, etc.) and those who do not respond to the usual medications.
In summary, microscopic colitis is a relatively common cause of chronic watery diarrhoea that appears to be increasing in incidence over time. Whether the increase in incidence is real or a result of diagnostic bias due to increased use of colonoscopy with biopsy in patients with diarrhoea is unknown. However, microscopic colitis is more common than previously recognised, and is particularly common in elderly patients. Collagenous colitis, but not lymphocytic colitis, is more common in women, and in our experience, lymphocytic colitis is more common than collagenous colitis. The strengths of our study include the population based nature of the cohort, the review of all previous diagnoses by a single expert gastrointestinal pathologist, the review of all prior biopsies done for the evaluation of diarrhoea in county residents and the length of time over which the study was conducted, going back in time to soon after these forms of colitis were first described. The main weakness is the primarily Caucasian ethnic makeup of our county which limits generalisability to other ethnic or racial groups.
Acknowledgments
Presented in part at the 105th Annual Meeting of the American Gastroenterological Association, New Orleans, LA, 15–20 May 2004 and published in abstract form (Gastroenterology 2004;126(Suppl 2):A124).
Footnotes
Funding: Funded in part by an American College of Gastroenterology Clinical Research grant and a career development grant from the Miles and Shirley Fiterman Foundation. The Rochester Epidemiology Project is supported by grant AR 30582 from the National Institutes of Health, US Public Health Service.
Competing interests: None.
References
- 1.Read N W, Krejs G J, Read M G.et al Chronic diarrhea of unknown origin. Gastroenterology 198076264–271. [PubMed] [Google Scholar]
- 2.Lindstrom C G. “Collagenous colitis” with watery diarrhoea‐ a new entity? Pathologica Europa 19761187–89. [PubMed] [Google Scholar]
- 3.Pardi D S, Smyrk T C, Tremaine W J.et al Microscopic colitis: A review. Am J Gastroenterol 200297794–802. [DOI] [PubMed] [Google Scholar]
- 4.Raclot G, Queneau P E, Ottignon Y.et al Incidence of collagenous colitis. A retrospective study in the east of France. Gastroenterology 1994106A23 [Google Scholar]
- 5.Bohr J, Tysk C, Eriksson S.et al Collagenous colitis in Örebro, Sweden, an epidemiological study 1984–1993. Gut 199537394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olesen M, Eriksson S, Bohr J.et al Microscopic colitis: a common diarrhoeal disease. An epidemiological study in Örebro, Sweden, 1993–1998. Gut 200453346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez‐Banares F, Salas A, Forne M.et al Incidence of collagenous and lymphocytic colitis: a 5‐year population‐based study. Am J Gastroenterol 199994418–423. [DOI] [PubMed] [Google Scholar]
- 8.Agnarsdottir M, Gunnlaugsson O, Orvar K B.et al Collagenous and lymphocytic colitis in Iceland. Dig Dis Sci 2002471122–1128. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez‐Banares F, Forne M, Esteve M.et al Collagenous colitis and lymphocytic colitis in Terrassa, Spain: an epidemiological study 1993–1996. Gastroenterology 1997112A15 [Google Scholar]
- 10.Melton L J. History of the Rochester Epidemiology Project. Mayo Clin Proc 199671266–274. [DOI] [PubMed] [Google Scholar]
- 11.Bergstralh E J, Offord K P, Chu C P.et alCalculating incidence, prevalence and mortality rates for Olmsted County, Minnesota: An update. Rochester, MN: Section of Biostatistics, Mayo Clinic, Technical Report Series No, 49 1992
- 12.Cox D R. Some simple approximate tests for Poisson variates. Biometrika 195340354–360. [Google Scholar]
- 13.McCullagh P, Nelder J A.Generalized linear models. New York: Chapman and Hall, Ltd, 1983
- 14.Kaplan E L, Meier P. Non‐parametric estimation from incomplete observations. J Am Stat Assoc 195853457–481. [Google Scholar]
- 15.Kalbfleisch J D, Prentice R L.The statistical analysis of failure time data. New York: John Wiley and Sons, 1980
- 16.Loftus E V, Silverstein M D, Sandborn W J.et al Crohn's disease in Olmsted County, Minnesota, 1940–93: incidence, prevalence, and survival. Gastroenterology 19981141161–1168. [DOI] [PubMed] [Google Scholar]
- 17.Loftus E V, Silverstein M D, Sandborn W J.et al Ulcerative colitis in Olmsted County, Minnesota, 1940–93: incidence, prevalence, and survival. Gut 200046336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beaugerie L, Pardi D S. Drug‐induced microscopic colitis: proposal for a scoring system and review of the literature. Aliment Pharmacol Ther 200522277–284. [DOI] [PubMed] [Google Scholar]
- 19.Fine K D, Seidel R H, Do K. The prevalence, anatomic distribution, and diagnosis of colonic causes of chronic diarrhea. Gastrointest Endosc 200051318–326. [DOI] [PubMed] [Google Scholar]