Abstract
γ-Secretase is a membrane-associated protease that cleaves within the transmembrane region of amyloid precursor protein to generate the C termini of the two Aβ peptide isoforms, Aβ40 and Aβ42. Here we report the detergent solubilization and partial characterization of γ-secretase. The activity of solubilized γ-secretase was measured with a recombinant substrate, C100Flag, consisting largely of the C-terminal fragment of amyloid precursor protein downstream of the β-secretase cleavage site. Cleavage of C100Flag by γ-secretase was detected by electrochemiluminescence using antibodies that specifically recognize the Aβ40 or Aβ42 termini. Incubation of C100Flag with HeLa cell membranes or detergent-solubilized HeLa cell membranes generates both the Aβ40 and Aβ42 termini. Recovery of catalytically competent, soluble γ-secretase critically depends on the choice of detergent; CHAPSO (3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonate) but not Triton X-100 is suitable. Solubilized γ-secretase activity is inhibited by pepstatin and more potently by a novel aspartyl protease transition-state analog inhibitor that blocks formation of Aβ40 and Aβ42 in mammalian cells. Upon gel exclusion chromatography, solubilized γ-secretase activity coelutes with presenilin 1 (PS1) at an apparent relative molecular weight of approximately 2.0 × 106. Anti-PS1 antibody immunoprecipitates γ-secretase activity from the solubilized γ-secretase preparation. These data suggest that γ-secretase activity is catalyzed by a PS1-containing macromolecular complex.
Amyloid precursor protein (APP) is a ubiquitous membrane-spanning (type 1) glycoprotein that undergoes a variety of proteolytic processing events (1). Sequential cleavage of APP by the β- and γ-secretases generate the N and C termini, respectively, of Aβ peptides that comprise amyloid plaques in brain parenchyma of patients with Alzheimer's disease. APP proteolysis by α-secretase occurs within the Aβ peptide domain, thereby precluding formation of the amyloidogenic peptides. The C termini of the Aβ peptides are heterogeneous. Peptides of 40 or 42 aa in length (Aβ40 and Aβ42, respectively) typically are generated. Aβ42 is more prone to aggregation than Aβ40 (2) and is the major component of amyloid plaque (3).
Cleavage of APP by γ-secretase is extraordinary because the scissile bond appears to be situated within a transmembrane domain. It is unclear as to whether the C termini of Aβ40 and Aβ42 are generated by a single protease with sloppy specificity or two distinct proteases. Recent studies suggest that γ-secretase also cleaves within the transmembrane region of Notch, thereby releasing the Notch intracellular domain, which controls crucial cell fate decisions during development (4). The effects of transition state analog aspartyl protease inhibitors on cellular production of amyloid peptides have suggested that γ-secretase is an aspartyl protease (5).
Presenilin 1 (PS1) and presenilin 2 are polytopic membrane proteins that are involved in γ-secretase-mediated processing of APP. Autosomal dominant inheritance of assorted mutations in the PS1 gene is the most common cause of familial early-onset Alzheimer's disease (6). These PS1 mutations lead to increased production of Aβ42 (7–9). Likewise, certain mutations in presenilin 2 cause familial early-onset Alzheimer's disease (10) and increased generation of Aβ42. Cultured isolated neurons from PS1-deficient mice exhibit reduced γ-secretase-mediated cleavage of APP (11). It was suggested that PS1 might influence trafficking of APP and/or γ-secretase or it might play a more direct role in proteolytic cleavage of APP. Directed mutagenesis of two conserved transmembrane-situated aspartates in PS1 recently was shown to inactivate γ-secretase activity in cellular assays, suggesting that PS1 is either a unique diaspartyl cofactor for γ-secretase or is itself γ-secretase, an intramembranous aspartyl protease (12).
Characterization of γ-secretase has been hampered by prior reliance on cellular assays of γ-secretase activity. In this report, we describe the detergent solubilization and partial characterization of a catalytically competent, PS1-containing γ-secretase complex.
Materials and Methods
γ-Secretase Inhibitor.
The preparation of {1S-benzyl-4R-[1-(1S-carbamoyl-2-phenylethylcarbamoyl)-1S-3-methyl-butylcarbamoyl]-2R-hydroxy-5-phenylpentyl}carbamic acid tert-butyl ester (L-685,458), an aspartyl protease transition state analog inhibitor, was similar to that described for the 2S-hydroxy epimer (13).
Electrochemiluminescence (ECL) Assay of Aβ Peptides.
The Aβ peptides were measured by ECL assays (14, 15) using a variety of anti-Aβ antibodies and an Origen 1.5 Analyzer (Igen, Gaithersburg, MD). The 4G8 murine mAb (Senetek PLC, Maryland Heights, MO) binds an epitope in the Aβ peptide (within amino acids 18–21) that is immediately distal to the α-secretase cleavage site. The G2–10 murine mAb (provided by K. Beyreuther, University of Heidelberg, Germany) binds the C terminus that is exposed after γ-secretase-mediated cleavage to generate amino acid 40 of the Aβ40 peptide (16). The FCA3542 rabbit antibody (provided by F. Checler, Institut de pharmacologie moléculaire et cellulaire du Centre National de la Recherche Scientifique, Valbonne, France) binds the C terminus that is exposed after γ-secretase-mediated cleavage to generate amino acid 42 of the Aβ42 peptide (17). The 4G8 mAb was biotinylated with biotin-LC-sulfo-N-hydroxysuccinimide-ester (Igen). The G2–10 and FCA3542 antibodies were ruthenylated with TAG-N-hydroxysuccinimide ester (Igen). Aβ(x-40) was detected with biotinylated 4G8 and ruthenylated G2–10. Aβ(x-42) was detected with biotinylated 4G8 and ruthenylated FCA3542.
Membrane Preparation and Detergent Solubilization.
HeLa S3 cells from the American Type Culture Collection were grown in bioreactors (Analytical Biological Services, Wilmington, DE) in 90% DMEM, 10% FBS, 2 mM glutamine, and 100 μg/ml each of penicillin and streptomycin. Frozen HeLa S3 cells were resuspended in buffer A (50 mM Mes, pH 6.0/5 mM MgCl2/5 mM CaCl2/150 mM KCl) containing complete protease inhibitor mixture (Boehringer Mannheim). The cells were broken by single-pass through a French press (Spectronic Instruments, Rochester, NY). Cell debris and nuclei were removed by centrifugation at 800 × g for 10 min. The supernatant solutions were centrifuged at 100,000 × g for 60 min. The ensuing pellets were resuspended in buffer A, and the centrifugation was repeated. The final membrane pellets were resuspended in buffer A to yield a protein concentration of approximately 12 mg/ml. All procedures were performed at 4°C. The membranes were stored at −70°C. Detergent solubilization of HeLa cell membranes (protein concentration, 2.5 mg/ml in buffer A) involved treatment with 1% CHAPSO (3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonate) for 60 min at 4°C and centrifugation at 100,000 × g for 60 min. The ensuing supernatant solution is defined as solubilized γ-secretase.
In Vitro γ-Secretase Assay.
A DNA fragment encoding amino acids 596–695 of the 695-aa isoform of APP (APP695) and the Flag sequence (DYKDDDDK) at the C terminus was generated by PCR amplification with suitably designed oligonucleotides and the APP695 cDNA. The Met that serves as the translation start site is residue 596 of APP695 (the P1 residue with respect to the β-secretase cleavage site). This DNA fragment was inserted into the prokaryotic expression vector pET2–21b (Novagen). The recombinant protein, C100Flag, was overproduced in Escherichia coli [strain BL21(DE3)] and purified by Mono-Q column chromatography (Amersham Pharmacia Biotech). C100Flag (1.7 μM) was incubated with cell membranes (0.5 mg/ml) in the presence of CHAPSO, CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate), or Triton X-100 (0, 0.125, 0.25, 0.5, or 1%) in buffer B (50 mM Pipes, pH 7.0/5 mM MgCl2/5 mM CaCl2/150 mM KCl) at 37°C. The reactions were stopped by adding RIPA (150 mM NaCl/1.0% NP-40/0.5% sodium deoxycholate/0.1% SDS/50 mM Tris⋅HCl, pH 8.0) and boiling for 5 min. The samples were centrifuged and the supernatant solutions were assayed for the Aβ peptides by ECL. The Aβ40- and Aβ42-related products from γ-secretase-mediated processing of C100Flag possess a Met at the N terminus and are thus defined as M-Aβ40 and M-Aβ42, respectively. Likewise, supernatant solution (0.125 mg/ml) from CHAPSO-extracted HeLa cell membranes (solubilized γ-secretase) was incubated with C100Flag (1.7 μM) in buffer B containing 0.25% CHAPSO and subsequently assayed for M-Aβ40 and M-Aβ42 by using ECL.
Mass Spectrometric Analysis of γ-Secretase-Mediated Products.
The products of the γ-secretase in vitro assay were captured with immobilized biotinylated G2–10 mAb and analyzed by surface-enhanced laser desorption/ionization time of flight MS (Ciphergen Biosystems, Palo Alto, CA) in the presence of EAM-1 matrix solution (Ciphergen Biosystems). External standards were used for calibration.
Characterization of Detergent Solubilized γ-Secretase Activity.
Varying concentrations of C100Flag (0–13.3 μM) were incubated with solubilized γ-secretase as described above, and M-Aβ40 formation was determined by ECL. The pH dependence of in vitro γ-secretase activity was determined by using pH-adjusted 50 mM Mes and 50 mM Pipes for the pH 5.0–6.5 and pH 6.5–9.0 ranges, respectively; otherwise, the assay for M-Aβ40 was as described above. Gel exclusion chromatography was performed as follows: solubilized γ-secretase was diluted 4-fold in buffer B and 1 ml was loaded onto a Superose 6 HR 10/30 column (Amersham Pharmacia Biotech) by using an AKTAexplorer chromatography system (Amersham Pharmacia Biotech). The column was eluted with buffer B containing 0.25% CHAPSO. Fractions (0.5 ml) were analyzed for in vitro γ-secretase activity as described above. The fractions were analyzed by SDS/PAGE/immunoblot analysis for the PS1 heterodimer by using an anti-PS1-N-terminal fragment (NTF) antibody (rabbit polyclonal antibody provided by C. Masters, University of Melbourne, Victoria, Australia) and an anti-PS1-C-terminal fragment (CTF) antibody (rabbit polyclonal antibody 29498, provided by K. Beyreuther and C. Elle, University of Heidelberg, Germany). Pepstatin (0–200 μM) was added to solubilized γ-secretase in the presence of CHAPSO (0.25%) and C100Flag in buffer B and incubated 90 min at 37°C, and the samples then were assayed for M-Aβ40 and M-Aβ42 as described above.
Immunoprecipitation of γ-Secretase Activity Using an Anti-PS1 Antibody.
Solubilized γ-secretase (2.5 mg/ml) was diluted 10-fold in buffer B and adjusted to 0.25% CHAPSO. The samples were treated overnight at 4°C with normal IgG (2.5 μg/ml) or rabbit affinity-purified polyclonal antibody to PS1-NTF (2.5 μg/ml; Ab14, provided by S. Gandy, Nathan Kline Institute, Staatsburg, NY; ref. 18). The samples were mixed with protein G-agarose beads overnight at 4°C and then centrifuged. The ensuing pellets were washed three times with buffer B, resuspended in buffer B containing 0.25% CHAPSO, and assayed for γ-secretase activity by ECL (measuring both M-Aβ40 and M-Aβ42). In addition, the pellets from the anti-PS1 and normal IgG immunoprecipitation steps were eluted with SDS sample buffer and analyzed for PS1-NTF and PS1-CTF by SDS/PAGE/immunoblot analysis using the Ab14 and 29498 antibodies, respectively.
Results
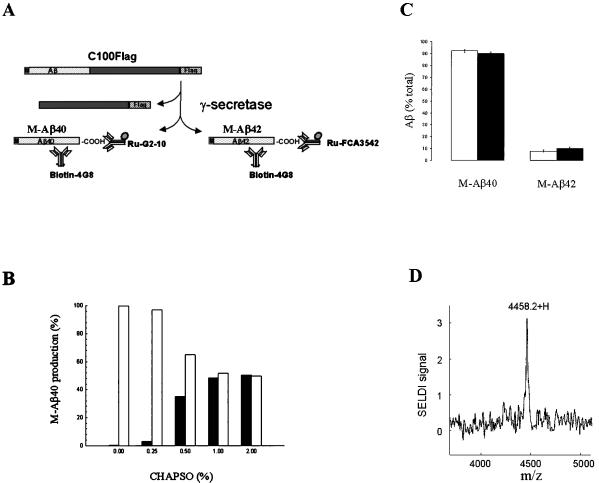
Cleavage of APP by α- or β-secretase appears to be a prerequisite for γ-secretase-mediated processing. Hence, our in vitro assay for γ-secretase activity uses a fusion protein, C100Flag, consisting of the β-CTF portion of APP (amino acids 596–695) with a Met and a Flag tag sequence at the N and C terminus, respectively (Fig. 1A). The Flag tag is a negatively charged peptide sequence that was incorporated to reduce substrate aggregation. Cleavage of C100Flag by γ-secretase should result in the generation of Aβ derivatives, M-Aβ40 and M-Aβ42, that possess an N-terminal Met and either the Aβ40 or Aβ42 termini, respectively (as illustrated in Fig. 1A). The cleavages are measured by ECL using derivatized anti-Aβ antibodies: biotinylated 4G8 and either ruthenylated G2–10 or FCA3542 (which specifically recognize the C termini of Aβ40 and Aβ42, respectively).
Figure 1.
In vitro γ-secretase assay. (A) Schematic representation of the fusion protein substrate, C100Flag, consisting sequentially of an N-terminal Met (M), APP597–695 (the Aβ domain is shown), and the Flag tag (Flag) sequence, and its processing by γ-secretase. The Aβ40- and Aβ42-related products (M-Aβ40 and M-Aβ42, respectively) are detected by ECL using biotinylated 4G8 antibody and the ruthenylated G2–10 or FCA3542 antibodies, respectively. (B) Solubilization of γ-secretase activity by extraction of cellular membranes with CHAPSO detergent. HeLa cell membranes were treated with the indicated CHAPSO concentration, and the samples were centrifuged. The supernatant solutions (filled bars) and resuspended pellets (open bars) were diluted in assay buffer to yield a uniform CHAPSO concentration (0.25%), incubated with C100Flag, and assayed for M-Aβ40 production by ECL. The γ-secretase activities are expressed as % of the total activity (supernatant solution plus pellet) at each detergent concentration. The data are the mean values of two independent experiments. (C) Generation of the Aβ40- and Aβ42-related products from C100Flag by HeLa cell membranes or solubilized γ-secretase. Intact membranes (500 μg/ml) (open bars) or solubilized γ-secretase (125 μg/ml) (filled bars) was incubated in the presence of C100Flag (1.7 μM) and CHAPSO (0.25%) at 37°C for 90 min. The reactions were quenched with RIPA and boiled. The resulting mixtures were centrifuged and the supernatant solutions were assayed for M-Aβ40 and M-Aβ42 by ECL. Standard curves using Aβ40 and Aβ42 were generated to measure production of the corresponding peptides. The data show the M-Aβ40 and M-Aβ42 levels as a % of total M-Aβ40 and M-Aβ42 (mean ± SD, n = 3). The total amount of the Aβ-related species was 556 fmol and 310 fmol for the intact membranes and solubilized γ-secretase, respectively. (D) Mass spectrometric confirmation of the identity of the Aβ40-related product (M-Aβ40) in the in vitro γ-secretase assay using solubilized γ-secretase and C100Flag. SELDI, surface-enhanced laser desorption/ionization.
Simple addition of C100Flag to membranes prepared from HeLa cells does not lead to substrate cleavage and generation of M-Aβ40. However, inclusion of CHAPSO or CHAPS during the incubation of HeLa cell membranes with C100Flag promotes cleavage at the γ-secretase scissile bond. Triton X-100 fails to potentiate in vitro γ-secretase activity. Optimal activity is observed in the presence of 0.25% CHAPSO or CHAPS; however, the ECL signal is approximately 3-fold higher with CHAPSO as compared with CHAPS. Higher concentrations of CHAPSO or CHAPS lead to a progressive decline in γ-secretase activity. Membranes prepared from human embryonic kidney cells (HEK293) or Chinese hamster ovary cells also process C100Flag in the presence of 0.25% CHAPSO to generate M-Aβ40 (data not shown).
The ability to solubilize γ-secretase from cellular membranes while retaining enzymatic activity would facilitate the characterization of this elusive protease. Our approach involved treatment of HeLa cell membranes with varying amounts of CHAPSO (up to 2.0%) followed by centrifugation (Fig. 1B). The supernatant solutions (solubilized fractions) and the pellets (membrane fractions) then were assayed for γ-secretase activity with the C100Flag substrate. More γ-secretase activity is recovered in the solubilized fraction, relative to the detergent-extracted membrane fraction, as the CHAPSO concentration is raised stepwise to 1.0%. The amounts of γ-secretase activity that are solubilized by 1.0% and 2.0% CHAPSO are comparable. There are corresponding decreases in the pellet-associated γ-secretase activity as a result of the CHAPSO extraction. Henceforth, the standard solubilization conditions uses 1.0% CHAPSO and the ensuing supernatant fraction is defined as solubilized γ-secretase.
Approximately 50% of the γ-secretase activity in the HeLa cell membranes is solubilized by this CHAPSO extraction protocol (Fig. 1B). The reason for our inability to solubilize all of the γ-secretase activity by using the CHAPSO extraction protocol remains to be established. It is important to note, however, that both the solubilized and residual membrane bound γ-secretase activities are inhibited by the γ-secretase inhibitor, L-685,458 (data not shown; compound described below). This observation suggests marked similarity, perhaps identity, between the γ-secretase-like proteases that differentially partition during the detergent extraction step. The total recovery of γ-secretase activity in the solubilized γ-secretase and residual pellet is approximately 50% greater than that observed with the intact membranes in the presence of 0.25% CHAPSO (data not shown).
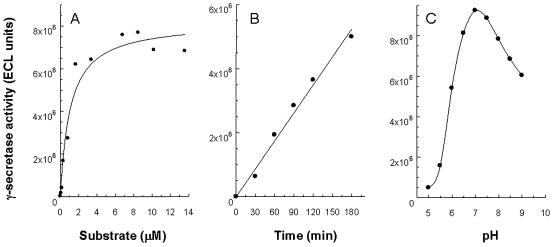
The activity of solubilized γ-secretase toward C100Flag was further characterized. The apparent Km for the processing of C100Flag to M-Aβ40 by solubilized γ-secretase is approximately 1 μM (Fig. 2A). Under the experimental conditions used, the time dependence of product formation is linear over a 3-h interval (Fig. 2B). The pH dependence of solubilized γ-secretase activity indicates a pH optimum of 7.0 (Fig. 2C). SDS/PAGE/immunoblot analysis of the in vitro assay samples using the 4G8 antibody shows that only a small fraction of C100Flag is consumed during the incubation period (data not shown).
Figure 2.
Characterization of detergent-solubilized γ-secretase activity toward C100Flag. (A) Dependence of M-Aβ40 formation on the C100Flag concentration. The data show the ECL signal after a 90-min incubation at 37°C. (B) Time dependence of M-Aβ40 formation by solubilized γ-secretase. The ECL signals are depicted. (C) pH dependence of solubilized γ-secretase activity. The ECL signals for the generation of M-Aβ40 are shown. The mean values from two independent experiments are shown.
Incubation of HeLa cell membranes or solubilized γ-secretase with C100Flag (both in the presence of 0.25% CHAPSO) also generates M-Aβ42 (Fig. 1C). However, considerably less M-Aβ42 is formed as compared with M-Aβ40 when assaying either source of γ-secretase. Interestingly, the Aβ42/Aβ40 cleavage ratios, approximately 0.1, with both intact membranes and solubilized γ-secretase are similar to the corresponding ratio that has been reported for Aβ peptides secreted into the conditioned media of cultured cells that are overexpressing APP (19). Our conclusion that cleavage is occurring at both γ-secretase scissile bonds in C100Flag is based on immunochemical evidence using antibodies that specifically recognize the Aβ40 and Aβ42 termini. The existence of the Aβ40-related cleavage was confirmed by surface-enhanced laser desorption/ionization-time of flight MS analysis (Fig. 1D). The experimentally determined value (4458.2) is virtually identical to the predicted value for M-Aβ40 (4460.1).
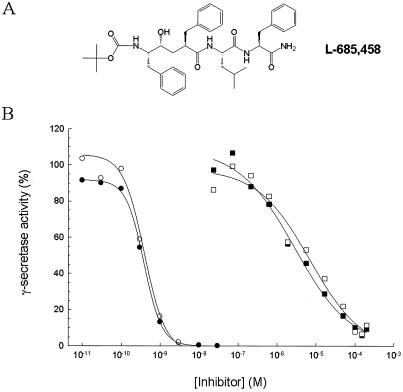
L-685,458 (Fig. 3A) is an aspartyl protease transition state analog that was identified (by M. S. Shearman, Merck Sharp & Dohme, Terlings Park, UK) as a γ-secretase inhibitor. The production of both M-Aβ40 and M-Aβ42 is blocked in our in vitro γ-secretase assay by L-685,458 with comparable inhibitory potencies, IC50 ≈ 0.3 nM (Fig. 3B). The findings that L-685,458 suppresses Aβ formation in HEK293 and Chinese hamster ovary cells that overexpress APP (M. S. Shearman, personal communication) as well as human postmitotic neuronal cells (NT2N) (X.-P.S., unpublished observation) provides crucial evidence that the processing of C100Flag by solubilized γ-secretase reflects bona fide γ-secretase activity. Pepstatin, a classical inhibitor of aspartyl class proteases, also blocked the production of M-Aβ40 and M-Aβ42 (Fig. 3B). The IC50 values of pepstatin for inhibiting the generation of M-Aβ40 and M-Aβ42 from C100Flag by solubilized γ-secretase are 4.0 and 5.9 μM, respectively.
Figure 3.
Inhibition of solubilized γ-secretase activity by L-685,458 and pepstatin. (A) Structure of L-685,458. (B) Evaluation of the inhibitory potencies of L-685,458 and pepstatin on solubilized γ-secretase activity. CT100Flag was added to solubilized γ-secretase containing the indicated concentrations of L-685,458 or pepstatin and incubated for 60 min. The ECL assay was used to detect M-Aβ40 (●) and M-Aβ42 (○) in the L-685,458-treated samples and M-Aβ40 (■) and M-Aβ42 (□) in the pepstatin-treated samples. The data are expressed as % of that observed in the absence of either L-685,458 or pepstatin. Shown are the mean values of two and five independent experiments with L-685,458 and pepstatin, respectively.
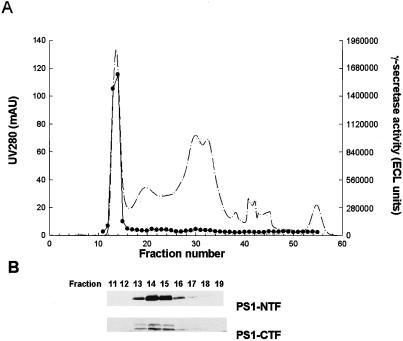
Solubilized γ-secretase prepared from HeLa cells was fractionated by gel exclusion chromatography. The A280 tracing is shown in Fig. 4A. The fractions were monitored for γ-secretase activity (production of M-Aβ40) using the in vitro γ-secretase assay (Fig. 4A). The γ-secretase activity eluted with an apparent relative molecular weight of 2 × 106. Enzymatic activity responsible for formation of M-Aβ42 from C100Flag eluted at the same position (data not shown). Varying the CHAPSO concentration from 0.1% to 0.5% does not alter the elution profile of the γ-secretase activity (data not shown). In light of the previously recognized close relationship between PS1 and γ-secretase activity, we evaluated the gel filtration fractions for the presence of PS1 by SDS/PAGE/immunoblot analysis (Fig. 4B). Interestingly, the fractions with γ-secretase activity (nos. 13–16) contain PS1 heterodimer as shown by immunodetection with antibodies versus PS1-NTF and PS1-CTF. SDS/PAGE/immunoblot analysis of the other fractions indicated that the PS1 heterodimer was absent from fractions devoid of γ-secretase activity (data not shown).
Figure 4.
Comigration of γ-secretase activity and PS1 during gel exclusion chromatography of solubilized γ-secretase. (A) Solubilized γ-secretase (1 ml) was chromatographed with Superose 6 gel filtration media. The A280 profile of the eluent is shown (dashed line). Fractions (0.5 ml) were collected and monitored for γ-secretase activity (M-Aβ40 production) with the in vitro γ-secretase assay using C100Flag (●). (B) Column fractions were evaluated for presence of PS1 by SDS/PAGE/immunoblot analysis using anti-PS1-NTF and anti-PS1-CTF antibodies.
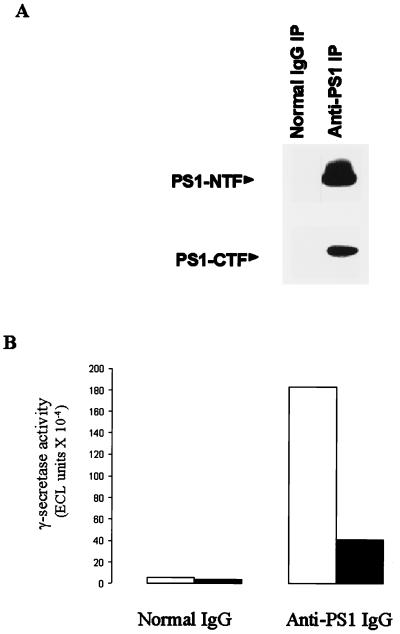
The coelution of PS1 and γ-secretase during gel exclusion chromatography prompted us to explore the partitioning behavior of γ-secretase activity during immunoprecipitation of PS1 using an anti-PS1-NTF antibody. Solubilized γ-secretase was subjected to immunoprecipitation using normal IgG or anti-PS1-NTF antibody. SDS/PAGE/immunoblot analysis revealed that PS1 was markedly reduced in the supernatant fraction from the treatment with immobilized anti-PS1-NTF antibody (data not shown). The pellet fractions from the immunoprecipitation with the anti-PS1-NTF antibody contained both PS1-NTF and PS1-CTF (Fig. 5A). Normal IgG did not sequester either PS1 fragment in the immobilized antibody pellet (Fig. 5A). Assay of the resuspended pellet fractions revealed the presence of γ-secretase activity when the anti-PS1-NTF antibody but not normal IgG was used for the immunoprecipitation (Fig. 5B). Both M-Aβ40 and M-Aβ42 were produced from C100Flag by the immunoabsorbed PS1 pellet. The γ-secretase activity that was captured by immobilized anti-PS1-NTF antibody was completely inhibited by L-685,458. Pretreatment of the anti-PS1-NTF antibody with its peptidic hapten blocked the appearance of γ-secretase activity in the pellet. The anti-PS1-NTF antibody used for the immunocapture attempt did not neutralize the solubilized γ-secretase activity. Future studies of the enzymatically active anti-PS1 immunoprecipitates, although possibly complicated by the presence of excess anti-PS1-NTF antibody, might enable the identification of other components in the γ-secretase complex.
Figure 5.
Binding of PS1 by immobilized anti-PS1 antibody results in capture of γ-secretase activity. Solubilized γ-secretase was subject to immunoprecipitation (IP) using normal IgG or anti-PS1-NTF IgG. (A) Western blotting analysis using anti-PS1-NTF and anti-PS1-CTF antibodies was performed with the immunoprecipitates. The bands corresponding to PS1-NTF and PS1-CTF are labeled. (B) The resuspended pellets from the normal IgG and anti-PS1 immunoprecipitation steps were evaluated with the in vitro γ-secretase assay (assaying for M-Aβ40 and M-Aβ42). The results are reported as ECL signals (mean of two independent assay determinations).
Discussion
Inhibition of γ-secretase is an appealing strategy to block the generation of the Aβ peptides and possibly thwart the onset and progression of Alzheimer's disease. Many key issues regarding γ-secretase have been intractable largely because of prior reliance on cellular assays for γ-secretase activity. Our ability to assay γ-secretase in its detergent-solubilized state now provides a valuable tool to help unravel the mysteries surrounding the catalytic activity/identity of this enigmatic protease. It is noteworthy that the in vitro γ-secretase assay using solubilized γ-secretase and C100Flag as substrate reproduces both major cleavage events (giving rise to the Aβ40 and Aβ42 termini) that are ascribed to γ-secretase activity in cells.
The ability to recover catalytically competent soluble γ-secretase critically depends on the choice of detergent used for the membrane extraction. CHAPSO and CHAPS yield active enzyme, whereas Triton X-100 does not. It previously was shown that the PS1 heterodimer can be extracted from cellular membranes by CHAPS detergent without causing dissociation of its constituent subunits (20). In contrast, the PS1 heterodimer is disrupted by Triton X-100 treatment. The parallels between the integrity of the PS1 heterodimeric structure and recovery of γ-secretase activity as measured with the in vitro assay suggests that the intact PS1 heterodimer is required for γ-secretase activity.
It remains to be determined whether one γ-secretase with sloppy specificity or two distinct γ-secretases are responsible for the generation of the C termini of Aβ40 and Aβ42. The differential effect of certain aldehyde-containing inhibitors on the production of Aβ40 and Aβ42 in cells fueled speculation that two different γ-secretases are involved (21). However, our observations that L-685,458 and pepstatin nonselectively inhibit formation of Aβ40 and Aβ42 in the in vitro assay, immunocaptured PS1 is able to catalyze both C-terminal-cleavage events, and the proteolytic activities that generate the Aβ40 and Aβ42 termini coelute during gel exclusion chromatography, together, suggest that a single γ-secretase may be responsible for both cleavage events. It was proposed (22) that the existence of more than one γ-secretase cleavage site may reflect disparate membrane thickness within the cell (as a result of varying cholesterol content). However, our solubilized γ-secretase preparation (which should be uniformly impacted by a coexisting detergent micelle) catalyzes both cleavage events. Indeed, the Aβ42/Aβ40 ratio is similar to that seen with cells. Additional studies are clearly required to understand the specificity determinants of the γ-secretase-mediated cleavage.
The similar partitioning of PS1 and γ-secretase activity during gel exclusion chromatography and immunoprecipitation (using an anti-PS1 antibody) provides biochemical evidence for linkage between PS1 and solubilized γ-secretase activity. At first glance, our studies appear to provide another line of evidence to support the hypothesis that PS1 is γ-secretase (12). However, as with the prior evidence, there are caveats that pose reasonable uncertainty regarding the assertion that PS1 and γ-secretase are one and the same. The elution profile of γ-secretase activity (as well as PS1) during gel exclusion chromatography is indicative of a high molecular weight catalytic entity that is substantially larger than the PS1 heterodimer (Mr ≈ 50,000). Hence, PS1 could be serving as a vital regulatory protein in the apparent γ-secretase macromolecular complex rather than the protease itself. Various reports have documented that PS1 is engaged in high molecular weight intracellular complexes; however, a linkage with γ-secretase activity has heretofore not been shown (20, 23). In any event, more definitive biochemical evidence (e.g., covalent labeling of PS1 by an active site directed inhibitor of γ-secretase) must be produced before the purported identity between PS1 and γ-secretase is firmly established. Likewise, further studies are required to identify the components of the putative γ-secretase complex and their role, if any, in catalysis.
Acknowledgments
We thank Drs. C. Elle and K. Beyreuther for kindly providing us with the anti-PS1-CTF antibody; Dr. S. Gandy and C. Masters for the anti-PS1-NTF antibodies; and Drs. M. Sherman and T. Harrison (Merck Sharp & Dohme, Terlings Park, U.K.) for contributing L-685,458.
Abbreviations
- APP
amyloid precursor protein
- PS1
presenilin 1
- NTF
N-terminal fragment
- CTF
C-terminal fragment
- ECL
electrochemiluminescence
- CHAPSO
3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonate
- L-685,458
{1S-benzyl-4R-[1-(1S-carbamoyl-2-phenylethylcarbamoyl)-1S-3-methyl-butylcarbamoyl]-2R-hydroxy-5-phenylpentyl}carbamic acid tert-butyl ester
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.110126897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.110126897
References
- 1.Selkoe D J. Trends Cell Biol. 1998;8:447–453. doi: 10.1016/s0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- 2.Jarrett J T, Berger E P, Lansbury P T., Jr Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 3.Kuo Y M, Emmerling M R, Vigo-Pelfrey C, Kasunic T C, Kirkpatrick J B, Murdoch G H, Ball M J, Roher A E. J Biol Chem. 1996;271:4077–4081. doi: 10.1074/jbc.271.8.4077. [DOI] [PubMed] [Google Scholar]
- 4.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm J S, Schroeter E H, Schrijvers V, Wolfe M S, Ray W J, et al. Nature (London) 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe M S, Xia W, Moore C L, Leatherwood D D, Ostaszewski B, Rahmati T, Donklor I O, Selkoe D J. Biochemistry. 1999;38:4720–4727. doi: 10.1021/bi982562p. [DOI] [PubMed] [Google Scholar]
- 6.Sherrington R, Rogaev E I, Liang Y, Rogaeva E A, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. Nature (London) 1995;375:754–760. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 7.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird T D, Hardy J, Hutton M, Kukull W, et al. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 8.Duff K, Eckman C, Zehr C, Yu X, Prada C M, Perez-Tur J, Hutton M, Buee L, Harigaya Y, Yager D, et al. Nature (London) 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 9.Borchelt D R, Thinakaran G, Eckman C B, Lee M K, Davenport F, Ratovitsky T, Prada C M, Kim G, Seekins S, Yager D, et al. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 10.Levy-Lahad E, Wijsman E M, Nemens E, Anderson L, Goddard K A B, Weber J L, Bird T D, Schellenberg G D. Science. 1995;269:970–973. doi: 10.1126/science.7638621. [DOI] [PubMed] [Google Scholar]
- 11.De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Nature (London) 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe M S, Xia W, Ostaszewski B L, Diehl T S, Kimberly W T, Selkoe D J. Nature (London) 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 13.DeSolms S J, Giuliani E A, Guare J P, Vacca J P, Sanders W M, Graham S L, Wiggins J M, Darke P L, Sigal I S, Zugay J A, et al. J Med Chem. 1991;34:2852–2857. doi: 10.1021/jm00113a025. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Leland J K, Yost D, Massey R J. Bio/Technology. 1994;12:193–194. doi: 10.1038/nbt0294-193. [DOI] [PubMed] [Google Scholar]
- 15.Khorkova O E, Patel K, Heroux J, Sahasrabudhe S. J Neurosci Methods. 1998;82:159–166. doi: 10.1016/s0165-0270(98)00053-3. [DOI] [PubMed] [Google Scholar]
- 16.Ida N, Hartmann T, Pantel J, Schröder J, Zerfass R, Förstl H, Sandbrink R, Masters C L, Beyreuther K. J Biol Chem. 1996;271:22908–22914. doi: 10.1074/jbc.271.37.22908. [DOI] [PubMed] [Google Scholar]
- 17.Barelli H, Lebeau A, Vizzavona J, Delaere P, Chevallier N, Drouot C, Marambaud P, Ancolio K, Buxbaum J D, Khorkova O, et al. Mol Med. 1997;3:695–707. [PMC free article] [PubMed] [Google Scholar]
- 18.Thinakaran G, Borchelt D R, Lee M K, Slunt H H, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, et al. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 19.Asami-Odaka A, Ishibashi Y, Kikuchi T, Kitada C, Suzuki N. Biochemistry. 1995;34:10272–10278. doi: 10.1021/bi00032a022. [DOI] [PubMed] [Google Scholar]
- 20.Capell A, Grünberg J, Pesold B, Diehlmann A, Citron M, Nixon R, Beyreuther K, Selkoe D J, Haass C. J Biol Chem. 1998;273:3205–3211. doi: 10.1074/jbc.273.6.3205. [DOI] [PubMed] [Google Scholar]
- 21.Citron M, Diehl T S, Gordon G, Biere A L, Seubert P, Selkoe D J. Proc Natl Acad Sci USA. 1996;93:13170–13175. doi: 10.1073/pnas.93.23.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tischer E, Cordell B. J Biol Chem. 1996;271:21914–21919. doi: 10.1074/jbc.271.36.21914. [DOI] [PubMed] [Google Scholar]
- 23.Yu G, Chen F, Levesque G, Nishimura M, Zhang D M, Levesque L, Rogaeva E, Xu D, Liang Y, Duthie M, et al. J Biol Chem. 1998;273:16470–16475. doi: 10.1074/jbc.273.26.16470. [DOI] [PubMed] [Google Scholar]