Abstract
Three defining clinical symptoms of autism are aberrant reciprocal social interactions, deficits in social communication, and repetitive behaviors, including motor stereotypies and insistence on sameness. We developed a set of behavioral tasks designed to model components of these core symptoms in mice. Male mice from ten inbred strains were characterized in assays for sociability, preference for social novelty, and reversal of the spatial location of the reinforcer in T-maze and Morris water maze tasks. Six strains, C57BL/6J, C57L/J, DBA/2J, FVB/NJ, C3H/HeJ, and AKR/J, showed significant levels of sociability, while A/J, BALB/cByJ, BTBR T+tf/J, and 129S1/SvImJ mice did not. C57BL/6J, C57L/J, DBA/2J, FVB/NJ, BALB/cByJ, and BTBR T+tf/J showed significant preference for social novelty, while C3H/HeJ, AKR/J, A/J, and 129S1/SvImJ did not. Normal scores on relevant control measures confirmed general health and physical abilities in all strains, ruling out artifactual explanations for social deficits. Elevated plus maze scores confirmed high anxiety-like behaviors in A/J, BALB/cByJ, and 129S1/SvImJ, which could underlie components of their low social approach. Strains that showed high levels of performance on acquisition of a T-maze task were also able to reach criterion for reversal learning. On the Morris water maze task, DBA/2J, AKR/J, BTBR T+tf/J, and 129S1/SvImJ failed to show significant quadrant preference during the reversal probe trial. These results highlight a dissociation between social task performance and reversal learning. BTBR T+tf/J is a particularly interesting strain, displaying both low social approach and resistance to change in routine on the water maze, consistent with an autism-like phenotype. Our multitask strategy for modeling symptoms of autism will be useful for investigating targeted and random gene mutations, QTLs, and microarray analyses.
Keywords: autism, locomotion, sociability, social preference, social approach, T-maze, Morris water maze, reversal tasks
1. Introduction
Autism is a neurodevelopmental disorder, defined in the DSM-IV by three fundamental symptoms [2]. Aberrant reciprocal social interactions include low levels of social approach, and qualitatively unusual modes of social interaction [44,96]. Deficits in social communication include delayed development of speech and poor expressive language [76]. Stereotyped, repetitive, and ritualistic behaviors, narrow restricted interests, insistence on sameness and resistance to change in habit are components of the third defining diagnostic [18, 120]. While evidence for neuropathology in autism suggests increased brain volume [6,8,30,37,61,62,94,97,126] and other neuroanatomical changes [7,32,78,95,103,127], and fMRI studies indicate reduced activation of the amgydala and fusiform gyrus during social tasks [43,92,105], there is no consistent neurological or biochemical marker for diagnosis, and the etiology of autism remains unknown. In addition, there is a lack of effective therapeutic strategies [119]. A significant genetic component for autism is supported by studies of concordance rates between identical twins [13,35,48,70,121], and candidate autism-susceptibility genes have been proposed from linkage and association analyses [11,27,45,87,93,98,129]. These advances in our understanding of the genetic basis of autism are leading to the development of promising mouse models that reflect genetic polymorphisms linked to autism [5,66].
One of the challenges in the evaluation and use of mouse models for autism is to design behavioral tests that reflect the core symptoms of the disease [66,85,88,100]. Without biological markers, behavioral traits with face validity to the core characteristics of autism represent one approach toward evaluating genetic contributions and potential treatments. We and other labs are engaged in developing mouse behavioral tasks with conceptual analogies to the three defining features of autism. The present study addresses the first symptom, low or aberrant social approach, and the third symptom, resistance to change in habit. The goal of the present experiments is to understand the genetic variability across inbred strains of mice on these tasks, which can then be used to identify genes in strains with unusual traits in these behavioral domains.
We have developed a mouse social approach task to assess sociability, the tendency to spend time with another conspecific, and preference for social novelty, the ability to discriminate and choose between familiar and new conspecifics [86,89]. In this procedure, the mouse is placed in the center compartment of a three-chambered test box, and given a choice between spending time in the side containing an unfamiliar (stranger) conspecific mouse, or remaining alone. The stranger mouse is contained within a small wire cage, to allow exposure to visual, auditory, olfactory, and some tactile stimuli, while preventing aggressive or sexual interactions. Measures taken during the test include time spent in each side, entries into each side, and time spent sniffing each cage. An identical wire cage in the opposite side chamber serves as a control novel object, to measure exploration of something new that has no social valence. Adult male mice of three standard inbred strains, C57BL/6J, DBA/2J, and FVB/NJ, and the F1 hybrid B6129, demonstrated a clear preference for spending time in the proximity of another mouse, versus in proximity to a novel object, while the A/J strain did not exhibit significant levels of sociability [86,89]. This social deficit in A/J may result from their general lack of active exploration and anxiety-like phenotype, as observed on the elevated plus maze [23,79,107,115]. Using a similar task, Brodkin and colleagues [24,102] have found low levels of social approach in mice from the BALB/cJ strain, which is also characterized by high levels of anxiety-like behaviors [12,36,40].
A second component of our social behavior task evaluates preference for social novelty in mice. In this phase of the test, a second unfamiliar mouse (stranger 2) is placed into the wire cage that was empty during the assessment of social approach. The test mouse then has a choice between spending time in the side with the now-familiar stranger 1, or investigating the newly-introduced stranger 2. C57BL/6J, DBA/2J, and FVB/NJ, but not A/J, showed significant preference for proximity to stranger 2, versus the already-investigated stranger 1 [86,89].
In addition to deficits in social interaction, children with autism can show cognitive inflexibility, as seen in restricted interests, rigid adherence to schedules, insistence on sameness, and upset at changes in routine and habit. Perseveration and reversal tasks in mice have reasonable face validity to components of these symptoms. We are using reversal learning in T-maze and water maze spatial tasks to examine resistance to change a learned pattern of behavior in mice. After reaching criterion on acquisition trials to learn the location of a food reward in the T-maze, or the location of the hidden escape platform in the water maze, the reinforcer location is switched to an opposite arm of the T-maze, or opposite quadrant of the water maze. Inbred strains of mice that fail to adapt to the new conditions for reinforcement may provide a model for the insistence on sameness characteristic of the autism phenotype.
The present study replicates and extends the mouse strain distribution on our social tasks to include six new inbred strains, C57L/J, C3H/HeJ, AKR/J, BALB/cByJ, BTBR T+tf/J, and 129S1/SvImJ, in comparison to C57BL/6J, DBA/J, FVB/NJ, and A/J. These strains were selected from the top tier of inbred mouse strains recommended by the Jackson Laboratory Mouse Phenome Project (http://aretha.jax.org/pub-cgi/phenome/mpdcgi). Young male mice were employed, for consistency with the approximately 4:1 ratio of boys to girls in autism [48,49,87]. After completion of social testing, these ten inbred mouse strains were evaluated for reversal learning in the T-maze and/or water maze tasks. Evaluation of general health, home cage behaviors, neurological reflexes, activity in an open field, motor coordination, olfactory ability, and anxiety-related behaviors on the elevated plus-maze were conducted to control for procedural abilities necessary for the social and reversal tasks.
2. Materials and methods
2.1 Animals
Twenty male mice from eight inbred strains, C57BL/6J, C57L/J, DBA/2J, FVB/NJ, AKR/J, A/J, BALB/cByJ, and 129S1/SvImJ, nineteen male mice from the C3H/HeJ strain, and 24 male mice from the BTBR T+tf/J strain were purchased from The Jackson Laboratory, Bar Harbor, ME (JAX). An additional set of ten male mice from the A/J strain (JAX) was independently tested for elevated plus maze performance. An additional set of twenty male mice from the AKR/J strain (JAX) was tested, due to health problems arising in the older mice (see section on Test procedures). Additional sets of C67BL/6J males were independently tested on the social task to confirm consistency of findings across time. Mice were 3 to 4 weeks of age upon arrival at the University of North Carolina animal facility in Chapel Hill, NC. Animals were housed separately by strain, with three to four mice per plastic tub cage, and provided with Purina 5058 chow and water ad libitum. The housing room was maintained at 23°C on a 12-h light/dark cycle (lights off at 7 PM). All procedures were conducted in strict compliance with the policies on animal welfare of the National Institutes of Health and the University of North Carolina (stated in the “Guide for the Care and Use of Laboratory Animals,” Institute of Laboratory Animal Resources, National Research Council, 1996 edition), and approved by the University of North Carolina Animal Care and Use Committee.
2.2. Test procedures
Order of testing for most strains was: 1) home cage observations at age 3–4 weeks; 2) general health and neurological reflexes at age 4–5 weeks; 3) open field locomotion and rotarod at age 5–6 weeks; 4) social behavior test at age 6–7 weeks; 5) olfactory latency to find buried food at age 7–8 weeks; 6) elevated plus-maze at age 8–10 weeks; 7) T-maze learning and reversal at age 3–4 months; 8) Morris water maze spatial learning and reversal at age 4–6 months. Unless otherwise indicated, testing was conducted under fluorescent laboratory lighting. Three strains (FVB/NJ, C3H/HeJ, and A/J) were tested for olfactory ability before, and not after, the social behavior test. The BTBR T+tf/J mice were evaluated for T-maze learning both before and after the water maze test. The 129S1/SvImJ mice were not tested in the T-maze procedure. Mice appeared to be healthy at the conclusion of the testing sequence, with the exception of mice from the AKR/J strain. While the group remained healthy throughout the T-maze testing, eleven of the mice died or were euthanized due to weight loss before the start of the water maze testing, possibly due to the high rate of leukemia reported for this strain (e.g. 90). A separate group of 20 AKR/J mice were tested, using the sequence described above, but with water maze testing conducted at an earlier time point (age 3–4 months), rather than the T-maze test.
2.3. Home cage behaviors
During the first week in the animal facility, observations of grouped mice in their home cages were taken at three different time points: 8:00 AM, 12:00 noon, and 6:50 PM. Records were taken for twenty minutes at each time point, for a total of 60 minutes of home cage observation. Two hours before the noon observation, one white cotton nestlet square (Ancare Corp., Bellmore, N.Y.) was added to each cage, in order to assess nest-building behavior. The evening observation was conducted ten minutes before lights off, and then for another ten minutes after the lights had gone off, using red light illumination. Records were taken for nestlet shredding, nest building, sleeping in huddles, activity, fighting, and any aberrant behaviors, such as tremor or seizures.
2.4. General health and neurological reflexes
Behavioral testing began one week after arrival into the animal facility. The mice were first evaluated for general health [38,39,86], including body weight, appearance of the fur and whiskers, body posture, and normality of gait. Reflexive reactions to a gentle touch from a cotton swab to the whiskers on each side of the face, the approach of the cotton swab to the eyes, and the sound from a metal clicker (Preyer reflex) were assessed. Animals were observed for the visual placing reflex (forepaw extension when lowered toward a visible surface), and for ability to grasp a metal grid with forepaws and hindpaws.
2.5. Locomotion
Exploratory activity in a novel environment was assessed in one 5-min test in a photocell-equipped automated open field (40 cm x 40 cm x 30 cm; Versamax system, Accuscan Instruments). Parameters included horizontal activity, ambulation (total distance traveled), fine movements (repeated breaking of the same set of photobeams), rearing movements, and time spent in the center region of the chamber. Testing was conducted in the morning or early afternoon, during the light phase of the mouse light/dark cycle. Activity chambers were contained inside sound-attenuating boxes, equipped with houselights and fans.
2.6. Rotarod performance
Mice were assessed for balance and motor coordination on an accelerating rotarod (Ugo-Basile, Stoelting Co., Wood Dale, Il). Revolutions per minute (rpm) were set at an initial value of 3, with a progressive increase to a maximum of 30 rpm across the five-minute test session. Each animal was given a single test session consisting of two trials, with 45 seconds between each trial. Latency to fall, or to rotate off the top of the turning barrel, was measured by the rotarod timer. If the mouse immediately fell off at the beginning of the first trial, that trial was not counted, and the mouse was given a new trial. Records were taken on the number of mice that inverted (clung to the barrel for a full rotation) during each trial for every strain except FVB/NJ.
2.7. Sociability and preference for social novelty
The social behavior apparatus, illustrated in Figure 1A and previously described [89], was designed to assess whether subject mice tend to spend time with stranger mice. The apparatus was a rectangular, three-chambered box fabricated from clear polycarbonate. Dividing walls had retractable doorways allowing access into each chamber. Photocells were embedded in each doorway to allow automatic quantification of entries and duration in each chamber of the social test box. The chambers of the apparatus were cleaned with water and dried with paper towels between each trial. At the end of each test day, the apparatus was sprayed with 70% ethanol and wiped clean with paper towels.
Figure 1.

Testing apparatus for (A) social behavior, (B) an appetitively-motivated T-maze learning task, and (C) spatial learning in the Morris water maze. In the preference for social novelty task (A), the subject mouse has a choice between staying in the center chamber, spending time in the side chamber with stranger 1, or spending time in the side chamber with a newly introduced conspecific, stranger 2 (each enclosed in a wire cage). In the T-maze procedure (B), the mouse is trained to enter either the left or right arm to receive a food reward. In the water maze task (C), the subject mouse is shown on the submerged escape platform. Multiple cues in the room are used for spatial navigation to locate the hidden platform.
2.7.1. Procedures for the social behavior test
A) Habituation. The test mouse was first placed in the middle chamber and allowed to explore for ten minutes, with the doorways into the two side chambers open. Each of the two sides contained an empty wire cage (Galaxy Cup, Spectrum Diversified Designs, Inc., Streetsboro, Ohio). The wire cages were 11 cm in height, with a bottom diameter of 10.5 cm and bars spaced 1 cm apart. A weighted cup was placed on the top of each cage to prevent climbing by the test mice. Each wire cage was used only once per day, and all cages were washed with soap and water at the end of each test day. B) Sociability. After the habituation period, the test mouse was enclosed in the center compartment of the social test box, and an unfamiliar mouse (stranger 1; a C57BL/6J male), further described below, was enclosed in one of the wire cages and placed in one of the side chambers. The location for stranger 1 alternated between the left and right sides of the social test box across subjects. Following placement of stranger 1, the doors were re-opened, and the subject was allowed to explore the entire social test box for a ten-minute session. Measures were taken of the amount of time spent in each chamber and the number of entries into each chamber by the automated testing system. In addition, a human observer scored time spent sniffing each wire cage, using a computer keypad and software developed by Dr. Josephine M. Johns, University of North Carolina, Chapel Hill, NC, and Dr. Larry W. Means, East Carolina University, Greenville, NC [67]. C) Preference for social novelty. At the end of the ten-minute sociability test, each mouse was further tested in a third ten-minute session to quantitate preference to spend time with a new stranger. A new unfamiliar mouse was placed in the wire cage that had been empty during the previous ten-minute session. The test mouse had a choice between the first, already-investigated, now-familiar mouse (stranger 1) and the novel unfamiliar mouse (stranger 2). As described above, measures were taken of the amount of time spent in each chamber, the number of transitions between chambers of the apparatus, and time spent sniffing each wire cage.
2.7.2. Controls for the social behavior test
To confirm the absence of a side preference bias for either of the two side chambers of the social test box, measures were taken of time spent in each side during the 10-minute habituation period. None of the strains showed a significant preference for either the right or left side [no main effect of side; p>0.05, within-group repeated measures analysis for each strain]. In addition, separate groups of C57BL/6J mice were periodically evaluated in the social behavior task, to confirm that the environmental parameters for the assay had not changed and normal tendencies for social approach could still be observed. Stranger mice were adult male C57BL/6J (JAX), and were housed in cages separate from and distant to the cages housing the subject mice, to avoid visual, auditory, and olfactory contact. Strangers had no previous physical contact with the subjects, and were kept in a separate location from the subjects on the day of testing. Several days before the start of social testing, the mice serving as strangers were habituated to the wire cages in the social apparatus for 5–10 minutes per day, for at least five days. Each stranger was used only once per day, and the strangers for the sociability test and the social novelty tests were taken from separate cages. Containing the stranger mouse in a wire cage served the purpose of preventing aggressive and sexual interactions, as well as ensuring that all social approach was initiated only by the subject mouse. Previous experiments indicated that the strain of the stranger did not change the social approach of the subject [89]. The empty wire cage served as a control for the properties of the container, in addition to serving as the novel inanimate object with no social valence.
2.8. Olfactory test
Several days before the olfactory test, an unfamiliar food (Froot Loops, Kellogg Co., Battle Creek, MI) was placed overnight in the home cages of the subject mice, in order to avoid food neophobia on the day of testing. Observations of consumption were taken to ensure that the novel food was palatable to the mice. In most cases, mice immediately began eating the cereal. Cages were also checked for uneaten cereal on the following day. On the day of the test, each mouse was placed in a large, clean tub cage (46 cm L x 23.5 cm W x 20 cm H), containing 3 cm deep paper chip bedding (Canbrands Product,
Moncton NB, Canada), and allowed to explore for five minutes. The animal was removed from the cage, and one Froot Loop was buried in the cage bedding, approximately 1 cm below the surface of the litter. The subject mouse was then returned to the cage for a fifteen minute test. Measures were taken of latency to find the buried food. Mice from the A/J, C3H/HeJ, and FVB/NJ strains were tested without food deprivation. Because latencies were sometimes long, and food restriction is routinely used to shorten latencies in buried food testing, the remaining inbred strains (C57BL/6J, C57L/J, DBA/2J, AKR/J, BALB/cByJ, BTBR T+tf/J, and 129S1/SvImJ) were tested for olfactory ability following a period of food deprivation. For these groups, all food was removed from the home cage sixteen to twenty hours before the test.
2.9. Elevated plus-maze test for anxiety-like behaviors
This conflict test is based on a natural tendency of mice to actively explore a new environment, versus the aversive properties of an elevated open runway [12,47,74]. In the present study, mice were given one five-minute trial on the plus-maze, which had two closed arms, with walls 40 cm in height, and two open arms. The maze was elevated 50 cm from the floor, and the arms were 21 cm long. Animals were placed on the center section (9.5 cm x 9.5 cm), and allowed to freely explore the maze. Measures were taken of time on, and number of entries into, the open and closed arms. Percent open arm time was calculated as 100 x (time spent on the open arms/(time in the open arms + time in the closed arms)). Percent open arm entries was calculated using the same formula, but using the measure for entries.
2.10. T-maze acquisition and reversal learning
Mice were first food-deprived to 85–90% of their free-feeding body weight before starting the appetively-motivated T-maze task. Mice were habituated to the T-maze (Figure 1B) and shaped to obtain food from cups recessed into the ends of the arms across five days. Ten training trials per day were then initiated. For each mouse, one arm was designated as the correct arm. One reinforcer (Noyes sucrose pellet, 20 mg., Research Diets, Inc., New Brunswick, NJ) was available in the designated arm for each trial. The reinforced arm was on the left side for half of the mice, and on the right side for the other half. At the beginning of each test session, the mouse was placed in the start box at the bottom of the T-maze stem. The start box door was opened, and the mouse was given a choice between entering either arm. If the mouse made the correct choice, it was given time to consume the sugar pellet, and then guided back into the start box for the next trial. Incorrect choices were not rewarded or punished. For each successive trial, the reward was always placed in the same arm. Latency to enter an arm, number of errors in arm selection, and number of days to criterion were recorded by a human observer. The criterion for task acquisition, determined for each mouse, was 80% correct responses on 3 consecutive days for the following strains: C57BL/6J, C57L/J, FVB/NJ, C3H/HeJ, AKR/J, and A/J. Each mouse that met criterion for acquisition was then further tested using a reversal procedure, in which the reinforcer location was switched to the arm opposite to its previous location for each mouse. Ten trials per day were then administered for reversal learning, using the same methods and criterion as described above.
Criterion was changed for two inbred strains: BALB/cByJ and BTBR T+tf/J, to limit the number of days of training. Instead of running each mouse until criterion had been met, a group average for 80% correct responses across 3 days of testing was used. When the group average was at criterion, the mice were further tested using a reversal procedure. For the DBA/2J mice, a criterion of 70% correct responses on 3 consecutive days was used because results from the first inbred strain tested, the C57BL/6J mice, suggested that the 80% criterion level was too difficult. Since the water maze procedure (described below) was determined to be more useful in finding differences in reversal learning, the last strain, 129S1/SvImJ, was not evaluated with the T-maze test.
2.11. Water maze test
The Morris water maze task, illustrated in Figure 1C, was based on the standard methods for spatial learning in rodents [84,91,130]. The water maze consisted of a large circular pool (diameter = 122 cm) partially filled with water (45 cm deep, 24–26°C), located in a room with numerous visual cues. To allow detection by an automated tracking system (Noldus Ethovision), overhead fluorescent lighting was used for dark-pigmented strains, while halogen lighting directed at the ceiling was used for the albino strains (A/J, AKR/J, and BALB/cByJ). Mice were tested for their ability to find an escape platform (diameter = 12 cm) on three different components: visible platform acquisition, hidden (submerged) platform acquisition, and subsequent probe trial in the absence of the platform, followed by hidden platform training in a new location and subsequent probe trial for reversal learning. In each case, the criterion for learning was an average latency of 15 seconds or less to locate the platform across a block of four consecutive trials per day.
In the visible platform test, each animal was given four trials per day, across three days, to swim to an escape platform cued by a patterned cylinder extending above the surface of the water. For each trial, the mouse was placed in the pool at one of four possible locations (randomly ordered), and then given 60 seconds to find the visible platform. If the mouse found the platform, the trial ended, and the animal was allowed to remain 10 seconds on the platform before the next trial began. If the platform was not found, the mouse was placed on the platform for 10 seconds, and then given the next trial. Measures were taken of latency to find the platform, swimming distance, and swimming velocity, via an automated tracking system (Noldus Ethovision). Only groups that were able to reach criterion with a visible platform were given further tests with the hidden platform. The visible platform test was not repeated for reversal learning.
The following week, mice were trained on the hidden platform test. Using the same procedure as described above, each animal was given four trials per day, for up to nine days, to learn the location of the submerged platform. At the end of the day that the group met the 15 second criterion for learning, or else on day nine of testing, mice were given a one-minute probe trial in the pool with the platform removed. In this case, selective quadrant search was evaluated by measuring percent of time spent in each quadrant of the pool. Spatial learning was demonstrated by greater swim times in the quadrant where the platform had been previously located, in comparison to other areas of the pool. In the week following the acquisition phase, mice were tested for reversal learning, using the same procedure. In this phase, the hidden platform was located in a different quadrant in the pool, diagonal to its previous location. As before, measures were taken of latency to find the platform, swimming distance, and swimming velocity. On the day that the criterion for learning was met, or else on day nine of testing, the platform was removed from the pool, and the group was given a probe trial to evaluate reversal learning.
2.12. Statistical analysis
Each inbred strain was tested separately in the behavioral assays. Therefore, data from each strain were analyzed separately, using within-strain comparisons relevant to the behavioral parameter(s) of the specific task. Data from the two components of the social behavior test (sociability and social novelty) were analyzed using within-strain repeated measures ANOVAs, with the factor of chamber side (e.g., stranger 1 side or the opposite side). Within-strain repeated measures ANOVAs were used to compare time spent in each quadrant of the water maze during the probe trials. For all comparisons, significance was set at p < 0.05.
3. Results
3.1. Home cage behaviors, neurobehavioral reflexes, sensory abilities, and motor functions
Preliminary observations indicated that the mice from the ten inbred strains appeared in good general health, without any overt impairments or aberrant responses. Table 1 describes the results of specific measures of general health, home cage behaviors, neurological reflexes, sensory abilities, and motor functions. At the initiation of testing, the majority of the inbred strains had body weights in the range of 19–21 grams, with the lowest average body weight observed for the C57L/J (C57L) strain. In the home cage, mice from each strain built nests from Nestlet squares, slept together in huddles, and did not display any unusual levels of activity or fighting during the home cage observation periods. Essentially all mice displayed normal vision on the forepaw visual placing reflex, and normal hearing on the Preyer acoustic startle reflex. Olfaction appeared to be normal in all strains, as individuals were able to locate a buried food reward, providing evidence that the strains were not anosmic. Five of the strains (C57BL/6J (C57BL), C57L, DBA/2J (DBA), FVB/NJ (FVB), and BALB/cByJ (BALB)) found the cereal within five minutes of testing. The longest latencies were observed in the C3H/HeJ (C3H) and A/J mice, which were among the three strains that did not have food deprivation before the test. All of the strains showed some level of proficiency in the accelerating rotarod task for motor coordination and balance, with the AKR/J (AKR) and 129S1/SvImJ (129) mice having the greatest ability, and the C3H and BTBR T+tf/J (BTBR) animals demonstrating lower levels of performance.
Table 1.
Physical characteristics, vocalizations, home cage behavior, sensory reflexes, olfactory ability, and a motor test. Data shown are means ± SEM for weight and latency measures, percent of cages for home cage measures, and percent of mice for other measures.
| C57BL/6J | C57L/J | DBA/2J | FVB/NJ | C3H/HeJ | AKR/J | A/J | BALB/cByJ | BTBR | 129S1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Physical Characteristics | ||||||||||
| Body weight (g) | 20.1±0.5 | 15±0.3 | 18.8±0.4 | 19.6±0.3 | 19.8±0.3 | 19.9±0.5 | 18.4±0.5 | 20.9±0.3 | 20±0.8 | 19.5±0.3 |
| Poor coat condition | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 8% | 0% |
| Piloerection | 0% | 0% | 6% | 35% | 5% | 0% | 0% | 15% | 4% | 0% |
| Vocalization during | ||||||||||
| handling or reflex test | 15% | 26% | 67% | 0% | 0% | 90% | 0% | 30% | 4% | 10% |
| Home cage (% cages) | ||||||||||
| Nest building | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| Huddling | 100% | 100% | 100% | 80% | 100% | 100% | 100% | 100% | 100% | 80% |
| Aberrant responses | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| Reflexes (% of mice normal) | ||||||||||
| Corneal | 85% | 100% | 78% | 100% | 100% | 100% | 100% | 90% | 80% | 100% |
| Visual placing | 100% | 100% | 100% | 100% | 100% | 80% | 80% | 100% | 100% | 100% |
| Vibrissae orienting | 100% | 100% | 83% | 100% | 100% | 100% | 100% | 95% | 100% | 100% |
| Preyer reflex | 90% | 90% | 100% | 100% | 100% | 100% | 90% | 80% | 92% | 100% |
| Olfaction test | ||||||||||
| Uncovered buried food (% mice) | 85%* | 100%* | 100%* | 100% | 60% | 80%* | 70% | 100%* | 83%* | |
| 80%* | ||||||||||
| Latency to find (sec) | 282±67 | 167±32 | 158±34 | 198±46 | 639±72 | 409±70 | 423±81 | 84±15 | 379±89 | 337±72 |
| Motor coordination | ||||||||||
| Rotarod latency (sec) | 118±16 | 98±11 | 127±7 | 129±16 | 51±9 | 195±19 | 102±9 | 117±10 | 69±15 | 177±19 |
| Inverting on rotarod | ||||||||||
| Moy et al. (% mice, Trial 2) | 65% | 40% | 65% | Not recorded | 100% | 50% | 85% | 35% | 25% | 35% |
Mice were tested for olfactory ability following 16–20 hours of food deprivation.
Strain distributions for exploration in a novel environment are shown in Figure 2. Rank orders of the strain means for total distance traveled were BTBR T+tf/J > FVB/NJ > C57L/J > AKR/J > 129S1/SvImJ > DBA/2J > C57BL/6J > C3H/HeJ > BALB/cByJ > A/J. As previously described [22,56,80,107,112], A/J had extremely low levels of distance traveled, rearing movements, and time spent in the center of the open field. The 129 group had higher levels of horizontal activity and distance, but almost no rearing across the five-minute test. Interestingly, A/J and 129 mice appeared normal on the accelerating rotarod task, indicating that these strains are not generally impaired on motor tasks. The highest levels of rearing were seen in the FVB and C57L strains. These strains also showed the highest levels of center time in the open field.
Figure 2.

Activity in a novel open field environment in ten inbred mouse strains. Data shown are mean (+ SEM) for each strain for a five-minute test.
3.2. Elevated plus-maze
The elevated plus-maze test was used to investigate whether high levels of anxiety-like responses were associated with low social approach in the social behavior test. Figure 3 shows the percentage of session time spent on the open arms, and the percentage of the total entries into the open arms of the plus-maze, as well as the total number of entries, for eight inbred strains. Rank order of the strain means for % time in the open arms was C57L/J > BTBR T+tf/J > C57BL/6J > A/J > AKR/J > 129S1/SvImJ > BALB/cByJ > DBA/2J, generally consistent with the literature [12]. The artifactually high percentage of entries into the open arms in the A/J mice reflected the very low number of total entries for this strain.
Figure 3.
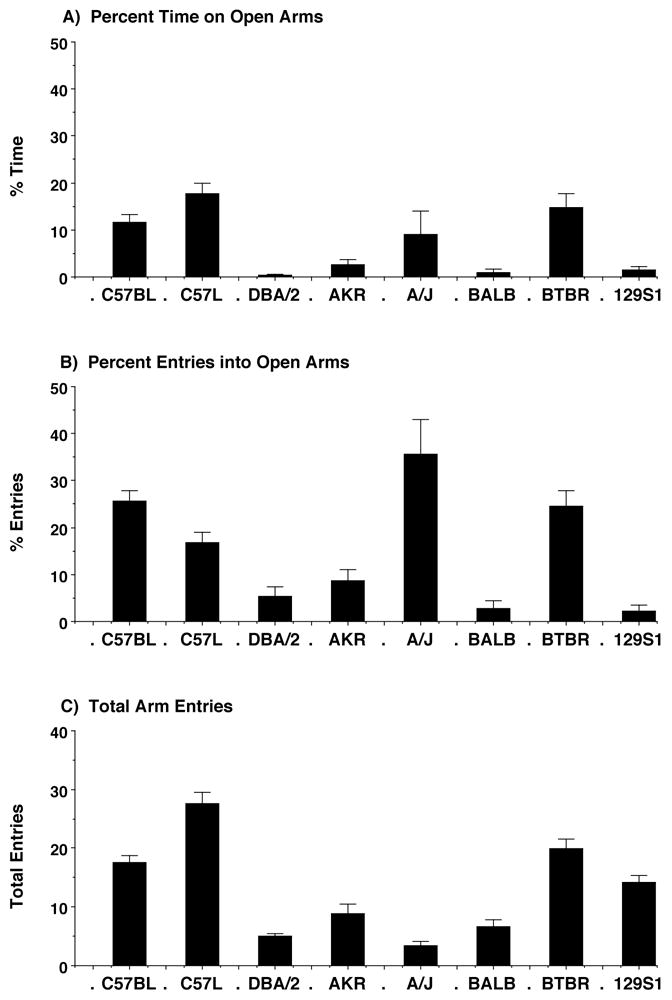
Performance in the elevated plus maze test for anxiety-like behavior in eight inbred mouse strains. Data shown are mean (+ SEM) for each strain for a five-minute test.
3.3. Social behavior test
Figure 4 presents the strain distributions for duration of time spent in each chamber for the sociability and preference for social novelty tests. Data from the DBA and FVB strains were taken from a previously published dataset using identical methods [86], and included for comparison to the other inbred mouse strains. Six of the inbred strains spent more time in the side containing the unfamiliar stranger 1, versus the side containing the empty wire cage [within-group repeated measures ANOVA, p<0.05]. Four inbred strains did not spend significantly more time with stranger 1: A/J, BALB, BTBR, and 129. Rank order of strain means for time spent with stranger 1 was: C3H/HeJ > DBA/2J > FVB/NJ > C57L/J > C57BL/6J > 129S1/SvImJ > BTBR T+tf/J > AKRJ > A/J > BALB/cByJ. On preference for social novelty, six of the strains had a significant preference for spending time in the side containing the newly-introduced stranger 2, in comparison to the side with stranger 1 [within-group repeated measures ANOVA, p<0.05]. Mice from the C3H/HeJ, AKR/J, A/J, and 129S1/SvImJ strains did not spend significantly more time with stranger 2 than with stranger 1. Rank order of strain means for time spent with stranger 2 was: BTBR T+tf/J > FVB/NJ > C57L/J > DBA/2J > 129S1/SvImJ > C3H/HeJ > C57BL/6J > AKRJ > A/J > BALB/cByJ.
Figure 4.

Duration of time spent in each chamber during the test for (A) sociability and (B) preference for social novelty in ten inbred mouse strains. Data shown are mean (+ SEM) for each strain for a ten-minute test. * p < 0.05, within-group comparison, time spent in proximity to stranger 1 is significantly different from time spent in proximity to a novel empty wire cage side (A), or time spent in proximity to stranger 2 is significantly different from time spent in proximity to stranger 1 side (B).
Only one strain, A/J, failed to demonstrate higher levels of sniffing for the cage containing stranger 1 in comparison to the empty cage (Figure 5). In contrast, five strains did not show a significant preference for social novelty on the sniffing measure: C3H, AKR, A/J, BTBR, and 129. The DBA and FVB strains are not included in this figure, because these groups were tested before methods were in place for quantifying sniff time.
Figure 5.

Time spent sniffing each cage during the test for (A) sociability and (B) preference for social novelty in eight inbred mouse strains. Data shown are mean (+ SEM) for each strain. * p < 0.05, within-group comparison, different from measure from the empty cage (A) or stranger 1 cage (B).
As previously reported, the number of entries did not reflect time spent in the side chambers, but appeared to be an independent measure of general exploratory locomotion [86,89]. Consistent with their low open field activity, A/J displayed low numbers of total entries on both the sociability and preference for social novelty tasks. It is interesting to note that BALB, BTBR, and 129, which showed social deficits, had numbers of entries comparable to other strains, such as DBA and C3H, that demonstrated high sociability. General exploration of the side chambers, therefore, appears to be independent of social approach tendencies, instead providing a control measure for motor abilities, general exploration, and/or anxiety-related traits.
3.4. Reversal learning in the T-maze task
The appetitively-motivated T-maze task was used to assess whether any of the inbred strains showed a resistance to change a learned pattern of behavior. Strains that acquired the task demonstrated good reversal learning (Figure 7). Large differences in the rates of acquisition, measured as the percent of each group meeting criterion, were observed for the first six inbred strains tested in the T-maze. One unpredicted finding was the poor original acquisition in C57BL. Only seven out of twenty C57BL mice reached the criterion of 80% correct responses across three days of testing. This finding was replicated in a second set of C57BL mice, where only three of twenty animals met criterion across fifteen days of testing. Low rates of acquisition were also observed in the related C57L strain, with only one of nineteen mice reaching criterion by day ten of testing (data not shown). The failure to learn was not due to a lack of reinforcer palatability, since mice consumed the reward on most correct trials, and was not due to low exploration in the T-maze. The subjects continued to make arm choices during each trial, but without forming a preference for the arm containing the reward. However, of the seven C57BL mice from the first group tested that met criterion for acquisition, all reached criterion on reversal learning.
Figure 7.

Acquisition and reversal learning in a T-maze task for eight inbred mouse strains. For the first six strains (panels A-F), only mice which learned the task during the acquisition phase were further tested for reversal learning. Criterion was set as 8 or more correct responses across 3 days, with 10 trials per day, except for the DBA/2J group, which had a criterion of 7 or more correct responses across 3 days, with 10 trials per day. For the last two strains (panels G and H), criterion was set as a group average of 8 or more correct responses across 3 days, with 10 trials per day. In these two groups, all of the subjects tested for acquisition were also tested for reversal learning (N = 20 BALB/cByJ and 24 BTBR T+tf/J mice).
For the BALB and BTBR strains, the criterion for learning was changed to a group average (rather than an individual average) of 8 or more correct responses across three days, with a maximum of ten days of testing (Figure 7G and 7H; number of correct trials across training days). Mice from both strains demonstrated rapid acquisition of the task, and high levels of reversal learning. Another issue in the development of the present sequence of autism-related tasks was the order of testing on the T-maze and Morris water maze, since both tasks are somewhat stressful. The BTBR strain was tested twice in the T-maze, first approximately one month before the Morris water maze, and then approximately two weeks following training in the Morris water maze. The BTBR mice demonstrated similar levels of acquisition and learning for the second T-maze test (data not shown) as seen in the first test (Figure 7H), indicating that the T-maze task can be conducted either before or after the Morris water maze task.
3.5. Reversal learning in the water maze task
The Morris water maze test was a second method used to assess resistance to change in a learned pattern of behavior across inbred strains. On the visible platform task, the C57BL, C57L, DBA, AKR, BALB, BTBR, and 129 strains had escape latencies ranging from 4.2 to 10 seconds by the third day of training. Three other strains, A/J and the visually-impaired FVB and C3H strains, failed to meet criterion on this task, and were not further tested. The A/J mice were observed to have low swim speeds, and showed floating and pawing at the walls of the water maze. The FVB and C3H mice did not appear to have difficulties in swimming, but tended to remain near the maze walls. C3H mice also demonstrated circling during some trials.
All strains reached criterion on the original hidden platform acquisition within seven days, except BALB and BTBR (Figure 8). The rank order for days to criterion for each strain was BTBR T+tf/J > C57BL/6J = 129S1/SvImJ > DBA/2J = BALB/cByJ > C57L/J = AKR/J. All strains reached criterion for reversal in acquisition of the new hidden platform location, with the rank order for days to criterion during reversal learning BALB/cByJ > BTBR T+tf/J = 129S1/SvImJ > C57BL/6J > C57L/J = DBA/2J = AKR/J.
Figure 8.

Acquisition and reversal in the Morris water maze task for seven inbred mouse strains. Mice were given up to nine days to reach criterion for learning, set at a group average latency of 15 seconds or less to find the hidden platform. After reaching criterion on the original acquisition, the location of the hidden platform was changed to a new quadrant for the reversal task. Data shown are mean (± SEM) of four trials per day.
During the probe trials, the platform was removed from the water maze, and measures of time in each quadrant were taken (Figure 9). After the original acquisition, all strains except DBA showed significant selective search on the probe trial [main effect of quadrant, within-strain repeated measures ANOVA, p<0.05]. Rank order for mean percent time spent in the trained quadrant was BALB/cByJ > C57L/J > 129S1/SvImJ > BTBR T+tf/J > C57BL/6J > AKR/J > DBA/2J. After re-training on the new hidden platform location, selective search on the probe trial for the reversal task was significant for C57BL, C57L, and BALB [main effect of quadrant, within-strain repeated measures ANOVA, p<0.05], and not significant for DBA, AKR, BTBR, and 129. Rank order for time spent in the trained quadrant in the reversal task was BALB/cByJ > C57L/J > C57BL/6J > 129S1/SvImJ > AKR/J > BTBR T+tf/J > DBA/2J.
Figure 9.

Selective quadrant search on the Morris water maze following (A) hidden platform training and (B) reversal of hidden platform location. Each mouse was given a one-minute probe trial with the escape platform removed. Target (black bars) indicates the quadrant where the platform has been located during training trials. * p<0.05, within-strain repeated measures ANOVA, significant main effect of quadrant.
4. Discussion
Modeling the symptoms of autism in mice presents a unique challenge. Poor language skills, idiosyncratic responses to sensory stimuli, absence of empathy and Theory of Mind, and lack of eye contact are a few examples of human symptoms [18,44,76,96,120] that are extremely difficult to parallel in mice. However, to investigate hypotheses about genes underlying autism, and to evaluate potential treatments, the field needs good behavioral tasks relevant to at least a subset of the more amenable symptoms of autism. We began with two core symptoms, aberrant social interaction and resistance to change in routine, because the natural repertoire of mice includes high levels of social interaction [46,85,116,117] and some examples of perseveration [53,54,99]. Our three-chambered automated social approach task was based on existing social tasks for rodents [17,42,46,69]. The present apparatus is a modification that restricts initiation of social approach to the subject mice only, to allow more selective scoring of the sociability trait in subject mice. The T-maze and Morris water maze reversal tasks were based on existing literature on learning and memory in rats and mice [1,31,104], and were conceptualized as relevant to the inability of autistic people to break habits and change routines. The present studies reveal the usefulness of these social and reversal tasks in explicating genetic backgrounds in mice that underlie unusually low levels of social approach and poor ability to learn a new task.
The ten inbred strains were selected from the forty mouse strains included in the Mouse Phenome Project, a comprehensive phenotype database administered by The Jackson Laboratory [19]. This database provides information on multiple strain characteristics, including anatomical measurements, behavioral profiles, and drug responses, as well as data on genetic background (http://aretha.jax.org/pub-cgi/phenome/mpdcgi). The strains in the present study were chosen to represent a wide range of genetic backgrounds. Most of the strains were also categorized as “high priority” for phenotyping by the Mouse Phenome Project, due to widespread use as experimental subjects, and as background strains for transgenic and null-allele mouse lines.
Some of the strains selected for the present project have known sensory or motoric deficiencies. The genotypes of the C3H/HeJ and FVB/NJ mice include the gene for retinal degeneration, which leads to blindness by the age of weaning [111]. This sensory impairment did not result in low sociability or the inability to perform the T-maze task in the present study, but did preclude testing for spatial learning in the Morris water maze. A/J mice are characterized by a mutation in the dysferlin gene, leading to a lack of dysferlin protein in skeletal muscle [65]. Similar deficiencies of dysferlin have been associated with muscular dystrophy in humans [e.g. 81]. We found that the A/J mice had markedly low levels of exploration and poor swimming ability in the water maze. Profiles for the AKR/J strain include high rates of leukemia, which leads to a shortened lifespan [e.g. 90]. Early mortality in the AKR/J group of the present study necessitated conducting the water maze test earlier in the testing sequence, using a second set of mice.
A thorough evaluation of the ten inbred strains was conducted to determine any physical, sensory, or motor defects that might interfere with their ability to perform the social and reversal tasks. Measures of general health, home cage behavior, body weight, olfactory ability to locate buried food, acoustic startle, visual forepaw placing, eye blink, ear twitch, whisker movement, open field activity, elevated plus-maze anxiety-related behaviors, and rotarod motor coordination and balance, were evaluated to rule out false positives that could lead to artifactual interpretations, as previously described for behavioral phenotyping of transgenic and knockout mice [16,39,110,118]. In addition, the reversal tasks included analysis of the original acquisition to evaluate learning ability independently of reversal.
Male mice from six inbred strains, C57BL/6J, C57L/J, DBA/2J, FVB/NJ, C3H/HeJ, and AKR/J, demonstrated significant sociability, as measured by the relative amount of time spent in the side of the test box containing an unfamiliar stranger. These results replicate and extend previous reports for social approach in mice [21,24,25,86,89,102]. Four inbred strains, A/J, BALB/cByJ, BTBR T+tf/J, and 129S1/SvImJ, did not spend more time with a new mouse, as compared to time spent with an inanimate novel object. However, tendencies for sociability could be seen in the measure for sniffing, which was significant for all but the A/J strain. The overall strain distribution indicates that sniffing at a cage containing another mouse was preferable to sniffing at an empty cage in almost every mouse strain tested in the present study, but this preference did not necessarily reflect the overall amount of time that the mice were spending in each side of the social test box. It is possible that sniffing provides a more sensitive measure for social approach, allowing the detection of sociability in strains such as BALB/cByJ or BTBR T+ tf/J. On the other hand, sniffing may reflect a generally-present investigatory strategy in mice, driven more by the richness and complexity of olfactory stimuli, and not by the social milieu per se. Our first methodological validation of this social approach task revealed a significant correlation between time spent in the chamber with a stranger mouse and time spent sniffing a stranger mouse (89). Further methodological analyses will be required to understand why some inbred strains showed a dissociation between time spent in the side chamber containing a stranger mouse and time spent in directly sniffing the wire cage containing a stranger mouse in the present experiments.
Low social approach in A/J, BALB/cByJ, and 129S1/SvImJ may represent an artifact of low exploratory activity, as seen in the open field test, and/or high anxiety-like behavior, as seen in the elevated plus-maze. A/J, BALB/cByJ, and 129/S1/SvImJ showed low levels of horizontal activity, total distance traveled, numbers of rears in the open field, and center time in the open field. Similarly, A/J, BALB/cByJ, and 129S1/SvImJ displayed low percent time and percent entries into the open arms of the elevated plus-maze. Further, low numbers of entries between chambers in the social apparatus were found for A/J, BALB/cByJ, and 129/S1/SvImJ.
A/J, BALB/cByJ, and related BALB/c substrains have been extensively reported to demonstrate more anxiety-like responses than other inbred mouse strains, dependent upon the behavioral assay used to test the mice [14,23,26,28,29,59,80,113]. Brodkin and colleagues [24,25,102], using a procedure similar to that of the present study, have reported specific deficiencies in social approach in the related BALB/cJ strain. Thus, anxiety-like traits, low exploration, and/or locomotor deficits may represent artifacts that would confound the interpretation of low social approach in these strains. Only one strain, BTBR T+tf/J, failed to show significant sociability while displaying high open field exploration and a lack of anxiety-like behavior on the elevated plus-maze.
Preference for social novelty characterizes many rodent species [15,17,42,46,69]. Comparison of time spent with a new stranger 2 versus time spent with the now-familiar stranger 1 in our three-chambered automated social approach apparatus conveys additional information about social recognition and social discrimination. The aberrant reciprocal social interactions in some autistic individuals include indiscriminate approach to strangers and acquaintances [51]. We reasoned that failure to display preference for social novelty by an inbred strain of mice would provide further support for a social deficit that may be relevant to the symptoms of autism. Preference for social novelty was detected in C57BL/6J, C57L/J, DBA/2J, FVB/NJ, and BALB/cByJ. Four strains, C3H/HeJ, AKR/J, A/J, and 129S1/SvImJ, failed to spend significantly more time in the chamber with the new stranger 2 than in the chamber of the more familiar stranger 1. These same four strains, as well as the BTBR T+tf/J strain, also failed to spent more time sniffing stranger 2, in comparison to stranger 1. BTBR T+tf/J was the only strain with significant preference for social novelty on time spent in the chamber with stranger 2, but no significant preference for social novelty on time spent sniffing stranger 2. This discrepancy between the measures may be related to the generally low rates of sniffing observed in the BTBR T+tf/J strain during the social novelty test. Low exploratory locomotion and/or high anxiety-like behaviors could again account for the lack of preference for social novelty in A/J and 129S1/SvImJ. AKR/J remains interesting as the only strain that appeared normal on all of the control measures, but did not display preference for social novelty. The rank orders for sociability did not match the rank orders for preference for social novelty in these ten inbred strains, indicating that these two social approach tasks are mediated by different background genes.
Two learning tasks were employed to evaluate the ability to change a response strategy to obtain reinforcement. Reversal of the food-reinforced arm of a T-maze, and reversal of the location of the hidden platform in the Morris water maze, were conceptualized as relevant to the impaired ability of autistic people to change their habits and routines. Acquisition of the original reinforced location served as a built-in control for procedural abilities to locomote, see, navigate, feed, swim, learn, and remember. Most of the strains demonstrated high levels of learning during both acquisition and reversal in the T-maze, including DBA/2J, FVB/NJ, C3H/HeJ, A/J, BALB/cByJ, and BTBR T+tf/J. All of the strains that were able to reach criterion during the visual cue task in the water maze were also able to reach criterion during acquisition and reversal of the hidden platform task.
Surprisingly, C57BL/6J and the related C57L/J strains showed poor performance on T-maze acquisition, while demonstrating high levels of ability in both the acquisition and reversal tests in the water maze. There have been similar conflicting accounts of maze learning in published reports of strain comparisons, with C57BL/6J mice showing a range of performance levels, dependent on the task and testing parameters, and the strains used for comparison [4,26,63,91,101,123]. In one study, mice from the BALB/cByJ and DBA/2J strains had better performance on a spatial T-maze task than mice from the C57BL/6J and A/J strains [41]. However, these group differences were not as evident with a non-spatial version of the T-maze task. Gerlai [55] reported that C57BL/6 mice have higher tendencies to explore a more-novel arm on the T-maze, in comparison to mice from the 129/SV, 129/SVEV, or DBA/2 strains. This type of strong bias for alternation would lead to lower levels of performance on tasks requiring the return to a previously-visited location, such as the T-maze task used in the present study.
Past work on strain distributions for water maze performance has suggested that BALB/cBy mice show low levels of spatial learning, especially in comparison to C57BL/6 or DBA/2J mice [50,91,114]. In contrast, Wahlsten and colleagues [123] reported excellent spatial learning, in terms of percent time in the target quadrant, for BALB/cByJ. Significant preference for the target quadrant was also observed for the BALB/cByJ strain in the present study
BTBR T+tf/J was the only strain tested that showed a specific deficit in reversal learning, without concomitant low scores for open field center time and plus-maze open arm time. The impairment was detected only in the Morris water maze, while BTBR T+tf/J was normal on reversal in the T-maze. In the water maze task, BTBR T+tf/J failed to show significant selective quadrant search on the reversal probe trial. Probe trial failure after normal hidden platform acquisition has been reported for targeted mutations of several genes [109,131], and generally indicates failure to form a hippocampal cognitive map of cues in the room environment. One interpretation of the reversal failure in BTBR T+tf/J is that this strain is deficient at making changes in its cognitive map of external environmental cues. Deficits in reversal learning in the Morris water maze have been observed in the Fmr1-null mouse, an animal model for fragile X syndrome [9,71]. These mice also show changes in social behavior [82,108]. Children with fragile X syndrome evidence a high rate of autistic-like behaviors [60], suggesting that the altered behavior in the mouse model may reflect an autism-like phenotype.
Interestingly, three of the four inbred strains not showing significant social approach, the BALB/cByJ, BTBR T+tf/J, and 129S1/SvImJ strains, have been characterized by varying degrees of hereditary corpus callosum agenesis [10,122,124,125]. A small percentage of BALB/c mice do not develop a corpus callosum, the thick band of axons that provides the interconnection between the two cerebral hemispheres of the brain [122,124,125]. Callosal deficiency is apparent, at much greater penetrance, in BTBR T+tf/J and some 129 substrains [10,124,125]. A survey of multiple inbred strains found that 100% of the BTBR T+tf/J mice had no corpus callosum, and most mice from that strain also had deficits in the hippocampal commissure [125]. It is noteworthy that clinical studies using magnetic resonance imaging or voxel-based morphometry have found reduced corpus callosum size in autistic patients [32,78,95,127], which may be related to symptom severity [64].
Recent work has provided evidence for abnormal social behavior in other genetic mouse models for autism. The Mecp2308/Y mouse models a loss of function mutation in the MECP2 gene, which causes Rett syndrome in humans [3]. This neurodevelopmental disorder is linked with autistic-like symptoms, such as severe language loss, motor stereotypies, and mental retardation [57]. The mouse model exhibits impaired social interactions and abnormal forelimb movements [83]. An aberrant behavioral phenotype, including decreased social approach and social interaction, can also be observed when the loss of Mecp2 occurs postnatally and is restricted to forebrain areas [52]. In human populations, susceptibility for autism spectrum disorders has been linked to chromosome 7q [33,128,129], which contains genes involved in early brain development. The function of one of these genes, WNT2, is mediated by dishevelled 1 (Dvl1). Mice with a loss of Dvl1 show less home cage huddling, but no changes in acquisition of spatial learning in the water maze test [73,75]. Another gene located in the 7q region is FOXP2. In humans, mutations in FOXP2 have been linked to severe language deficits [72,77]. Interestingly, disruptions of the Foxp2 gene in mice lead to overt reductions in the ultrasonic vocalizations that pups emit when isolated from their mothers [106]. The Foxp2-deficient mouse may provide a model for communication deficits early in development. Other mouse models that reflect neuroanatomical, biochemical, or genetic abnormalities associated with autism have been developed, and await systematic evaluation of their behavioral phenotype [34,85,88,99,100].
The present results support our multiple test strategy to evaluate mouse models for face validity to the symptoms of autism. It is unlikely that all of the defining and associated symptoms of autism will find parallels in a single inbred strain or knockout mouse. Instead, specific endophenotypes in autism offer specific targets for analogous phenotypes in an armamentarium of mouse models [58,68]. Behavioral endophenotypes that we and others have been able to model in mice include social approach for face validity to the core symptom of aberrant reciprocal social interactions, perseveration on reversal tasks for the core symptom of repetitive behaviors and resistance to change in routine, and anxiety-like responses on the elevated plus-maze and other tasks relevant to the commonly associated anxiety symptom. Measurements of brain volume in relevant mouse models may reflect the associated biological marker of larger brain size at young ages in autistic children [30,37,94,97]. Evaluating many control parameters of general health and physical abilities may reveal further relevant phenotypes in mouse models, such as hypersensitivity to sensory stimuli or sleep disorders. Further, conducting careful controls to evaluate general health will avoid false positive misinterpretations of phenotypes. The challenge to developing a good mouse model is to incorporate many features of autism without overinterpretation of irrelevant artifacts.
BTBR T+tf/J represents our first strong candidate strain, discovered from our initial analysis of 10 strains recommended by the International Mouse Phenome Project [19]. Unlike standard mouse strains, BTBR T+tf/J spent as much time with a novel object as with a stranger mouse, indicating a low level of sociability. Normal scores on measures of exploratory locomotion, olfaction, ability to discriminate stranger 1 from stranger 2 and preference for the new stranger, anxiety-like behaviors, sensory reflexes, and general health indicate a specific deficit in sociability in BTBR T+tf/J. Low sociability in A/J, BALB/cByJ, and 129S1/SvImJ are more difficult to interpret, since low social approach in these strains may have been confounded by low exploratory locomotion and high anxiety-like traits. Consistent with the present findings, Valerie Bolivar and Lorraine Flaherty at the Wadsworth Institute in Albany, New York have documented low reciprocal social interaction in BTBR T+tf/J [20,21]. Preliminary data by Hewlet McFarlane and Jacqueline Crawley at NIMH indicate low levels of social interactions in juvenile BTBR T+tf/J mice, as compared to juvenile C57BL/6J (manuscript in preparation).
Genetic analyses of interesting mouse strains, such as BTBR T+tf/J, by DNA microarrays and by mining single nucleotide polymorphism databases, may reveal the genes underlying low social approach, deficits in reversal learning, and other behavioral characteristics relevant to the autism phenotype. Identifying genes underlying both the behavioral deficits and neuroanatomical features, such as corpus callosum agenesis in BTBR T+tf/J, may highlight candidate genes to investigate for the neurodevelopmental pathology leading to autism. Obtaining information iteratively from mouse and human studies, using behavioral and genetic analyses, provides a novel approach to advancing knowledge about genes responsible for the fundamental neurodevelopmental defects and resulting behavioral symptoms in autism.
Figure 6.

Number of entries into each side chamber during the test for (A) sociability and (B) preference for social novelty in ten inbred mouse strains. Data shown are mean (+ SEM) for each strain. * p < 0.05, within-group comparison, entries into stranger 2 side different from entries into stranger 1 side.
Acknowledgments
The authors would like to thank Randal J. Nonneman for the photographs of the social test apparatus and water maze. Dr. Joseph Piven, Director of the University of North Carolina Autism Research Center, provided valuable insights throughout this project. Behavioral tests were conducted by the Mouse Behavioral Phenotyping Laboratory of the Neurodevelopmental Disorders Research Center, University of North Carolina. This work was supported by NIH STAART grant U54 MH66418 and NICHD grant P30 HD03110, and by the NIMH Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aggleton JP, Hunt PR, Nagle S, Neave N. The effects of selective lesions within the anterior thalamic nuclei on spatial memory in the rat. Behav Brain Res. 1996;81:189–198. doi: 10.1016/s0166-4328(96)89080-2. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Washington, DC: 1994. [Google Scholar]
- 3.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 4.Ammassari-Teule M, Hoffmann HJ, Rossi-Arnaud C. Learning in inbred mice: strain- specific abilities across three radial maze problems. Behav Genet. 1993;23(4):405–412. doi: 10.1007/BF01067443. [DOI] [PubMed] [Google Scholar]
- 5.Andres C. Molecular genetics and animal models in autistic disorder. Brain Research Bulletin. 2002;57(1):109–119. doi: 10.1016/s0361-9230(01)00642-6. [DOI] [PubMed] [Google Scholar]
- 6.Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59(2):175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- 7.Aylward EH, Minshew NJ, Goldstein G, Honeycutt NA, Augustine AM, Yates KO, Barta PE, Pearlson GD. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53:2145–2150. doi: 10.1212/wnl.53.9.2145. [DOI] [PubMed] [Google Scholar]
- 8.Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- 9.Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F, Vermey M, Bygrave A, Hoogeveen AT, Oostra BA, Reyniers E, De Boulle K, D'Hooge R, Cras P, van Velzen D, Nagels G, Martin J-J, De Deyn PP, Darby JK, Willems PJ. Fmr1 knockout mice: A model to study fragile X mental retardation. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- 10.Balogh SA, McDowell CS, Stavnezer AJ, Denenberg VH. A behavioral and neuroanatomical assessment of an inbred substrain of 129 mice with behavioral comparisons to C57BL/6J mice. Brain Res. 1999;836:38–48. doi: 10.1016/s0006-8993(99)01586-3. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett CW, Gharani N, Millonig JH, Brzustowicz LM. Three autism candidate genes: a synthesis of human genetic analysis with other disciplines. Int J Dev Neurosci. 2005;23(2–3):221–234. doi: 10.1016/j.ijdevneu.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 13.Bespalova IN, Buxbaum JD. Disease susceptibility genes for autism. Ann Med. 2003;35(4):274–281. doi: 10.1080/07853890310005966. [DOI] [PubMed] [Google Scholar]
- 14.Beuzen A, Belzung C. Link between emotional memory and anxiety states: a study by principle component analysis. Physio Behav. 1995;58(1):111–118. doi: 10.1016/0031-9384(95)00013-9. [DOI] [PubMed] [Google Scholar]
- 15.Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47(4):503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 16.Bilbo SD, Nelson RJ. Behavioral phenotyping of transgenic and knockout animals: a cautionary tale. Lab Anim (NY) 2001;30:24–29. [PubMed] [Google Scholar]
- 17.Bluthe RM, Gheusi G, Dantzer R. Gonadal steroids influence the involvement of arginine vasopressin in social recognition in mice. Psychoneuroendocrinology. 1993;18(4):323–335. doi: 10.1016/0306-4530(93)90028-j. [DOI] [PubMed] [Google Scholar]
- 18.Bodfish JW, Symons FJ, Parker DE, Lewis M. Varieties of repetitive behavior in autism: comparisons to mental retardation. J Autism Dev Disorders. 2000;30(3):237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- 19.Bogue MA, Grubb SC. The mouse phenome project. Genetica. 2004;122:71–74. doi: 10.1007/s10709-004-1438-4. [DOI] [PubMed] [Google Scholar]
- 20.Bolivar VJ, Flaherty L. Assessing autism-like behaviors in inbred strains of mice. Society for Neuroscience Abstract. 2003:318.13. [Google Scholar]
- 21.Bolivar and Flaherty, this volume [reference will be added before proofs go to press].
- 22.Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG. Genetic and behavioral differences among five inbred mouse strains commonly used in the production of transgenic and knockout mice. Genes Brain Behav. 2004;3:149–157. doi: 10.1111/j.1601-183x.2004.00064.x. [DOI] [PubMed] [Google Scholar]
- 23.Bouwknecht JA, Paylor R. Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav Brain Res. 2002;136:489–501. doi: 10.1016/s0166-4328(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 24.Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Brodkin et al., this volume [reference will be added before proof pages go to press].
- 26.Brooks SP, Pask T, Jones L, Dunnett SB. Behavioural profiles of inbred mouse strains used as transgenic backgrounds. II: cognitive tests. Genes Brain Behav. 2005;4:307–317. doi: 10.1111/j.1601-183X.2004.00109.x. [DOI] [PubMed] [Google Scholar]
- 27.Buxbaum JD, Silverman JM, Smith CJ, Greenberg DA, Kilifarski M, Reichert J, Cook EH, Jr, Fang Y, Song C-Y, Vitale R. Association between a GABRB3 polymorphism and autism. Molecular Psychia. 2002;7:311–316. doi: 10.1038/sj.mp.4001011. [DOI] [PubMed] [Google Scholar]
- 28.Carola V, Frazzetto G, Gross C. Identifying interactions between genes and early environment in the mouse. Genes Brain Behav. 2006;5:189–199. doi: 10.1111/j.1601-183X.2005.00152.x. [DOI] [PubMed] [Google Scholar]
- 29.Carola V, D’Olimpio F, Brunamonti E, Mangia F, Renzi P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res. 2002;134:49–57. doi: 10.1016/s0166-4328(01)00452-1. [DOI] [PubMed] [Google Scholar]
- 30.Carper RA, Courchesne E. Localized enlargement of the frontal cortex in early autism. Biol Psychiatry. 2005;57(2):126–133. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB, Morris RG. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- 32.Chung MK, Dalton KM, Alexander AL, Davidson RJ. Less white matter concentration in autism: 2D voxel-based morphometry. Neuroimage. 2004;23(1):242–251. doi: 10.1016/j.neuroimage.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 33.Collaborative Linkage Study of Autism. An autosomal genomic screen for autism. Am J Med Genet (Neuropsychiatr Genet) 1999;88:609–615. doi: 10.1002/(sici)1096-8628(19991215)88:6<609::aid-ajmg7>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 34.Connell S, Karikari C, Hohmann CF. Sex-specific development of cortical monoamine levels in mouse. Dev Brain Res. 2004;151:187–191. doi: 10.1016/j.devbrainres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60(5):524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 36.Cook MN, Williams RW, Flaherty L. Anxiety-related behaviors in the elevated zero-maze are affected by genetic factors and retinal degeneration. Behav Neurosci. 2001;115(2):468–476. [PubMed] [Google Scholar]
- 37.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290(3):337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 38.Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- 39.Crawley JN. Behavioral Phenotyping of Transgenic and Knockout Mice. New York: Wiley-Liss; 2000. What’s Wrong with My Mouse? [Google Scholar]
- 40.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 41.Crusio WE, Bertholet J-Y, Schwegler H. No correlations between spatial and non-spatial reference memory in a T-maze task and hippocampal mossy fibre distribution in the mouse. Behavioural Brain Res. 1990;41:251–259. doi: 10.1016/0166-4328(90)90112-r. [DOI] [PubMed] [Google Scholar]
- 42.Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37:49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- 43.Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dawson G, Webb S, Schellenberg GD, Dager S, Friedman S, Aylward E, Richards T. Defining the broader phenotype of autism: genetic, brain, and behavioral perspectives. Dev Psychopath. 2002;14:581–611. doi: 10.1017/s0954579402003103. [DOI] [PubMed] [Google Scholar]
- 45.Fatemi SH. Reelin mutations in mouse and man: from reeler mouse to schizophrenia, mood disorders, autism and lissencephaly. Molecular Psychia. 2001;6:129–133. doi: 10.1038/sj.mp.4000129. [DOI] [PubMed] [Google Scholar]
- 46.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nature Genetics. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 47.File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res. 2001;125:151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- 48.Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nature Rev Genetics. 2001;2:943–955. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- 49.Fombonne E. Epidemiological trends in rates of autism. Molecular Psychia. 2002;7:S4–S6. doi: 10.1038/sj.mp.4001162. [DOI] [PubMed] [Google Scholar]
- 50.Francis DD, Zaharia MD, Shanks N, Anisman H. Stress-induced disturbances in Morris water-maze performance: interstrain variability. Physiology Behav. 1995;58:57–65. doi: 10.1016/0031-9384(95)00009-8. [DOI] [PubMed] [Google Scholar]
- 51.Frith U. Autism and Asperger Syndrome. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- 52.Gemelli T, Berton O, Nelson ED, Perrotti LI, Jaenisch R, Monteggia LM. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biol Psychia. 2006;59:468–476. doi: 10.1016/j.biopsych.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 53.Gendreau PL, Petitto JM, Gariepy J-L, Lewis MH. D1 dopamine receptor mediation of social and nonsocial emotional reactivity in mice: effects of housing and strain difference in motor activity. Behav Neurosci. 1997;111(2):424–434. doi: 10.1037//0735-7044.111.2.424. [DOI] [PubMed] [Google Scholar]
- 54.Gendreau PL, Petitto JM, Gariepy J-L, Lewis MH. D2-like dopamine receptor mediation of social-emotional reactivity in a mouse model of anxiety: strain and experience effects. Neuropsychopharmacology. 1998;18(3):210–221. doi: 10.1016/S0893-133X(97)00131-0. [DOI] [PubMed] [Google Scholar]
- 55.Gerlai R. A new continuous alternation task in T-maze detects hippocampal dysfunction in mice: A strain comparison and lesion study. Behavioural Brain Research. 1998;95:91–101. doi: 10.1016/s0166-4328(97)00214-3. [DOI] [PubMed] [Google Scholar]
- 56.Gershenfeld HK, Neumann PE, Mathis C, Crawley JN, Li X, Paul SM. Mapping quantitative trait loci for open-field behavior in mice. Behav Genet. 1997;27:201–210. doi: 10.1023/a:1025653812535. [DOI] [PubMed] [Google Scholar]
- 57.Glaze DG. Rett syndrome: of girls and mice – lessons for regression in autism. Men Retard Dev Disabil Res Rev. 2004;10:154–158. doi: 10.1002/mrdd.20030. [DOI] [PubMed] [Google Scholar]
- 58.Gould TD, Gottesman II. Psychiatric endophenotypes and the development of valid animal models. Genes Brain Behav. 2006;5:113–119. doi: 10.1111/j.1601-183X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- 59.Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology. 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- 60.Hagerman RJ, Jackson AW, III, Levitas A, Rimland B, Braden M. An analysis of autism in fifty males with the fragile X syndrome. Am J Med Genetics. 1986;23:359–374. doi: 10.1002/ajmg.1320230128. [DOI] [PubMed] [Google Scholar]
- 61.Hardan AY, Minshew NJ, Mallikarjuhn M, Keshavan MS. Brain volume in autism. J Child Neurol. 2001;16:421–424. doi: 10.1177/088307380101600607. [DOI] [PubMed] [Google Scholar]
- 62.Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62(12):1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- 63.Heyser CJ, McDonald JS, Polis IY, Gold LH. Strain distribution of mice in discriminated Y-maze avoidance learning: genetic and procedural differences. Behav Neurosci. 1999;113(1):91–102. doi: 10.1037//0735-7044.113.1.91. [DOI] [PubMed] [Google Scholar]
- 64.Hrdlicka M, Dudova I, Beranova I, Lisy J, Belsan T, Neuwirth J, Komarek V, Faladova L, Havlovicova M, Sedlacek Z, Blatny M, Urbanek T. Subtypes of autism by cluster analysis based on structural MRI data. Eur Child Adolesc Psychiatry. 2005;14:138–144. doi: 10.1007/s00787-005-0453-z. [DOI] [PubMed] [Google Scholar]
- 65.Ho M, Post CM, Donahue LR, Lidov HGW, Bronson RT, Goolsby H, Watkins SC, Cox GA, Brown RH., Jr Disruption of muscle membrane and phenotype divergence in two novel mouse models of dysferlin deficiency. Hum Molecular Genetics. 2004;13(18):1999–2010. doi: 10.1093/hmg/ddh212. [DOI] [PubMed] [Google Scholar]
- 66.Insel TR. Mouse models for autism: report from a meeting. Mammalian Genome. 2001;12:755–757. doi: 10.1007/s00335-001-4006. [DOI] [PubMed] [Google Scholar]
- 67.Johns JM, Noonan LR, Zimmerman LI, McMillen BA, Means LW, Walker CH, Lubin DA, Meter KE, Nelson CJ, Pedersen CA, Mason GA, Lauder JM. Chronic cocaine treatment alters social/aggressive behavior in Sprague-Dawley rat dams and in their prenatally exposed offspring. Ann NY Acad Sci. 1998;846:399–404. [PMC free article] [PubMed] [Google Scholar]
- 68.Keller F, Persico AM. The neurobiological context of autism. Molecular Neurobiology. 2003;28:1–22. doi: 10.1385/MN:28:1:1. [DOI] [PubMed] [Google Scholar]
- 69.Knutson B, Panksepp J. Effects of serotonin depletion on the play of juvenile rats. Ann N Y Acad Sci. 1997;807:475–477. doi: 10.1111/j.1749-6632.1997.tb51942.x. [DOI] [PubMed] [Google Scholar]
- 70.Kolevzon A, Smith CJ, Schmeidler J, Buxbaum JD, Silverman JM. Familial symptom domains in monozygotic siblings with autism. Am J Med Genet B Neuropsychiatr Genet. 2004;129(1):76–81. doi: 10.1002/ajmg.b.30011. [DOI] [PubMed] [Google Scholar]
- 71.Kooy RF, D'Hooge R, Reyniers E, Bakker CE, Nagels G, De Boulle K, Storm K, Clincke G, De Deyn PP, Oostra BA, Willems PJ. Transgenic mouse model for the fragile X syndrome. American J Med Gen. 1996;64:241–245. doi: 10.1002/(SICI)1096-8628(19960809)64:2<241::AID-AJMG1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 72.Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- 73.Lijam N, Paylor R, McDonald MP, Crawley JN, Deng C-X, Herrup K, Stevens KE, Maccaferri G, McBain CJ, Sussman DJ, Wynshaw-Boris A. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- 74.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 75.Long JM, LaPorte P, Paylor R, Wynshaw-Boris A. Expanded characterization of the social interaction abnormalities in mice lacking Dvl1. Genes Brain Behav. 2004;3:51–62. doi: 10.1046/j.1601-183x.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 76.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule-Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 77.MacDermot KD, Bonora E, Sykes N, Coupe AM, Lai CS, Vernes SC, Vargha-Khadem F, McKenzie F, Smith RL, Monaco AP, Fisher SE. Identification of FOXP2 truncation as a novel cause of developmental speech and language deficits. Am J Hum Genet. 2005;76(6):1074–1080. doi: 10.1086/430841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manes F, Piven J, Vrancic D, Nanclares V, Plebst C, Starkstein SE. An MRI study of the corpus callosum and cerebellum in mentally retarded autistic individuals. J Neuropsychiatry Clin Neurosci. 1999;11(4):470–474. doi: 10.1176/jnp.11.4.470. [DOI] [PubMed] [Google Scholar]
- 79.Mathis C, Neumann PE, Gershenfeld H, Paul SM, Crawley JN. Genetic analysis of anxiety-related behaviors and responses to benzodiazepine-related drugs in AXB and BXA recombinant inbred mouse strains. Behav Genetics. 1995;25(6):557–568. doi: 10.1007/BF02327579. [DOI] [PubMed] [Google Scholar]
- 80.Mathis C, Paul SM, Crawley JN. Characterization of benzodiazepine-sensitive behaviors in the A/J and C57BL/6J inbred strains of mice. Behav Genetics. 1994;24(2):171–180. doi: 10.1007/BF01067821. [DOI] [PubMed] [Google Scholar]
- 81.Matsuda C, Aoki M, Hayashi YK, Ho MF, Arahata K, Brown RH., Jr Dysferlin is a surface membrane associated protein that is absent in Miyoshi myopathy. Neurology. 1999;53:1119–1122. doi: 10.1212/wnl.53.5.1119. [DOI] [PubMed] [Google Scholar]
- 82.Mineur YS, Huynh LX, Crusio WE. Social behavior deficits in the Fmr1 mutant mouse. Behav Brain Res. 2006;168:172–175. doi: 10.1016/j.bbr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 83.Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Molec Gen. 2005;14(2):205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- 84.Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal-lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 85.Moy SS, Nadler JJ, Magnuson TR, Crawley JN. Mouse models of autism spectrum disorders: the challenge for behavioral genetics. Am J Med Genetics. 2006;142(1):40–51. doi: 10.1002/ajmg.c.30081. [DOI] [PubMed] [Google Scholar]
- 86.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3(5):287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 87.Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113(5):472–486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- 88.Murcia CL, Gulden F, Herrup K. A question of balance: a proposal for new mouse models of autism. Int J Devl Neurosci. 2004;23(2–3):265–275. doi: 10.1016/j.ijdevneu.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 89.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson T, Crawley JN. Automated apparatus for rapid quantitation of autism-like social deficits in mice. Genes Brain Behav. 2004;3(5):303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 90.Ono T, Dean RG, Chattopadhyay SK, Cutler RG. Dysdifferentiative nature of aging: age-dependent expression of MuLV and globin genes in thymus, liver, and brain in the AKR mouse strain. Gerontology. 1985;31:362–372. doi: 10.1159/000212725. [DOI] [PubMed] [Google Scholar]
- 91.Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997;80(4):1087–1099. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- 92.Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social dysfunction in autism. Ment Retard Dev Disabil Res Rev. 2004;10(4):259–271. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- 93.Persico AM, D'Agruma L, Maiorano N, Totaro A, Militerni R, Bravaccio C, Wassink TH, Schneider C, Melmed R, Trillo S, Montecchi F, Palermo M, Pascucci T, Puglisi-Allegra S, Reichelt K-L, Conciatori M, Marino R, Quattrocchi CC, Baldi A, Zelante L, Gasparini P, Keller F for the CLSA. Reelin gene alleles and haplotypes as a factor predisposing to autistic disorder. Molecular Psychia. 2001;6:150–159. doi: 10.1038/sj.mp.4000850. [DOI] [PubMed] [Google Scholar]
- 94.Piven J, Arndt S, Bailey J, Andreasen N. Regional brain enlargement in autism: a magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 1996;35(4):530–536. doi: 10.1097/00004583-199604000-00020. [DOI] [PubMed] [Google Scholar]
- 95.Piven J, Bailey J, Ranson BJ, Arndt S. An MRI study of the corpus callosum in autism. Am J Psychiatry. 1997;154(8):1051–1056. doi: 10.1176/ajp.154.8.1051. [DOI] [PubMed] [Google Scholar]
- 96.Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader autism phenotype: evidence from a family history study of multiple-incidence autism families. Am J Psychiatry. 1997;154(2):185–190. doi: 10.1176/ajp.154.2.185. [DOI] [PubMed] [Google Scholar]
- 97.Piven J, Saliba K, Bailey J, Arndt S. An MRI study of autism: the cerebellum revisited. Neurology. 1997;49:546–551. doi: 10.1212/wnl.49.2.546. [DOI] [PubMed] [Google Scholar]
- 98.Polleux F, Lauder JM. Toward a developmental neurobiology of autism. Ment Retard Dev Disabil Res Rev. 2004;10(4):303–317. doi: 10.1002/mrdd.20044. [DOI] [PubMed] [Google Scholar]
- 99.Presti MF, Lewis MH. Striatal opioid peptide content in an animal model of spontaneous stereotypic behavior. Behav Brain Res. 2005;157:363–368. doi: 10.1016/j.bbr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 100.Rodier PM. Animal model of autism based on developmental data. Mental Retard Dev Dis Res Rev. 1996;2:249–256. [Google Scholar]
- 101.Rogers DC, Jones DNC, Nelson PR, Jones CM, Quilter CA, Robinson TL, Hagan JJ. Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of six inbred mouse strains. Behav Brain Res. 1999;105:207–217. doi: 10.1016/s0166-4328(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 102.Sankoorikal GMV, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 103.Saitoh O, Karns CM, Courshesne E. Development of the hippocampal formation from 2 to 42 years: MRI evidence of smaller area dentata in autism. Brain. 2001;124:1317–1324. doi: 10.1093/brain/124.7.1317. [DOI] [PubMed] [Google Scholar]
- 104.Schoenbaum G, Nugent S, Saddoris MP, Gallagher M. Teaching old rats new tricks: age-related impairments in olfactory reversal learning. Neurobiol Aging. 2002;23:555–564. doi: 10.1016/s0197-4580(01)00343-8. [DOI] [PubMed] [Google Scholar]
- 105.Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23(2–3):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 106.Shu W, Cho JY, Jiang Y, Zhang M, Weisz D, Elder GA, Schmeidler J, De Gasperi R, Sosa MAG, Rabidou D, Santucci AC, Perl D, Morrisey E, Buxbaum JD. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. PNAS. 2005;102(27):9643–9648. doi: 10.1073/pnas.0503739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singer JB, Hill AE, Nadeau JH, Lander ES. Mapping quantitative trait loci for anxiety in chromosome substitution strains of mice. Genetics. 2005;169(2):855–862. doi: 10.1534/genetics.104.031492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4(7):420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 109.Steiner RA, Hohmann JG, Holmes A, Wrenn CC, Cadd G, Jureus A, Clifton DK, Luo M, Gutshall M, Ma SY, Mufson EJ, Crawley JN. Galanin transgenic mice display cognitive and neurochemical deficits characteristic of Alzheimer's disease. PNAS. 2001;98(7):4184–4189. doi: 10.1073/pnas.061445598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tarantino LM, Gould TJ, Druhan JP, Bucan M. Behavior and mutagenesis screens: the importance of baseline analysis of inbred strains. Mammalian Genome. 2000;11:555–564. doi: 10.1007/s003350010107. [DOI] [PubMed] [Google Scholar]
- 111.The Jackson Laboratory. Genetic background effects: Can your mice see? JAX/Notes. 2002;485:2. [Google Scholar]
- 112.Thifault S, Lalonde R, Sanon N, Hamet P. Comparisons between C57BL/6J and A/J mice in motor activity and coordination, hole-poking, and spatial learning. Brain Res Bull. 2002;58:213–218. doi: 10.1016/s0361-9230(02)00782-7. [DOI] [PubMed] [Google Scholar]
- 113.Trullas R, Skolnick P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology. 1993;111:323–331. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- 114.Van Dam D, Lenders G, De Deyn PP. Effect of Morris water maze diameter on visual-spatial learning in different mouse strains. Neurobio Learn Mem. 2006;85:164–172. doi: 10.1016/j.nlm.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 115.van Gaalen MM, Stenzel-Poore MP, Holsboer F, Steckler T. Effects of transgenic overproduction of CRH on anxiety-like behaviour. Eur J Neurosci. 2002;15(12):2007–2015. doi: 10.1046/j.1460-9568.2002.02040.x. [DOI] [PubMed] [Google Scholar]
- 116.Van Loo PLP, de Groot AC, Van Zutphen BFM, Baumans V. Do male mice prefer or avoid each other’s company? Influence of hierarchy, kinship, and familiarity. J Applied Animal Welfare Sci. 2001;4(2):91–103. [Google Scholar]
- 117.Van Loo PLP, Van de Weerd HA, Van Zutphen LFM, Baumans V. Preference for social contact versus environmental enrichment in male laboratory mice. Lab Animals. 2004;38:178–188. doi: 10.1258/002367704322968867. [DOI] [PubMed] [Google Scholar]
- 118.van der Staay FJ, Steckler T. Behavioral phenotyping of mouse mutants. Behav Brain Res. 2001;125:3–12. doi: 10.1016/s0166-4328(01)00278-9. [DOI] [PubMed] [Google Scholar]
- 119.Volkmar FR. Pharmacological interventions in autism: theoretical and practical issues. J Clin Child Psychology. 2001;30(1):80–87. doi: 10.1207/S15374424JCCP3001_9. [DOI] [PubMed] [Google Scholar]
- 120.Volkmar FR, Lord C, Bailey A, Schultz RT, Klin A. Autism and pervasive developmental disorders. J Child Psychol Psychiatry. 2004;45(1):135–170. doi: 10.1046/j.0021-9630.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- 121.Volkmar FR, Pauls D. Autism. Lancet. 2003;362:1133–1141. doi: 10.1016/S0140-6736(03)14471-6. [DOI] [PubMed] [Google Scholar]
- 122.Wahlsten D. Deficiency of the corpus callosum: incomplete penetrance and substrain differentiation in BALB/c mice. J Neurogenet. 1989;5:61–76. doi: 10.3109/01677068909167265. [DOI] [PubMed] [Google Scholar]
- 123.Wahlsten D, Cooper SF, Crabbe JC. Different rankings of inbred mouse strains on the Morris maze and a refined 4-arm water escape task. Behav Brain Res. 2005;165:36–51. doi: 10.1016/j.bbr.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 124.Wahlsten D, Crabbe JC, Dudek BC. Behavioural testing of standard inbred and 5HT1B knockout mice: implications of absent corpus callosum. Behav Brain Res. 2001;125:23–32. doi: 10.1016/s0166-4328(01)00283-2. [DOI] [PubMed] [Google Scholar]
- 125.Wahlsten D, Metten P, Crabbe JC. Survey of 21 inbred mouse strains in two laboratories reveals that BTBR T/+ tf/tf has severely reduced hippocampal commissure and absent corpus callosum. Brain Res. 2003;971:47–54. doi: 10.1016/s0006-8993(03)02354-0. [DOI] [PubMed] [Google Scholar]
- 126.Waiter GD, Williams JHG, Murray AD, Gilchrist A, Perrett DI, Whiten A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. NeuroImage. 2004;22:619–625. doi: 10.1016/j.neuroimage.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 127.Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. Structural white matter deficits in high-functioning individuals with autistic spectrum disorder: a voxel-based investigation. Neuroimage. 2005;24(2):455–461. doi: 10.1016/j.neuroimage.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 128.Wassink TH, Brzustowicz LM, Bartlett CW, Szatmari P. The search for autism disease genes. Men Retard Dev Disabilities Res Rev. 2004;10:272–283. doi: 10.1002/mrdd.20041. [DOI] [PubMed] [Google Scholar]
- 129.Wassink TH, Piven J, Vieland VJ, Huang J, Swiderski RE, Pietila J, Braun T, Beck G, Folstein SE, Haines JL, Sheffield VC. Evidence supporting WNT2 as an autism susceptibility gene. Am J Med Genetics. 2001;105:406–413. doi: 10.1002/ajmg.1401. [DOI] [PubMed] [Google Scholar]
- 130.Wrenn CC, Kinney JW, Marriott LK, Holmes A, Harris AP, Saavedra MC, Starosta G, Innerfield CE, Jacoby AS, Shine J, Iismaa TP, Wenk GL, Crawley JN. Learning and memory performance in mice lacking the GAL-R1 subtype of galanin receptor. Eur J Neurosci. 2004;19:1384–1396. doi: 10.1111/j.1460-9568.2004.03214.x. [DOI] [PubMed] [Google Scholar]
- 131.Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]


