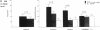Abstract
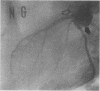
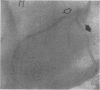
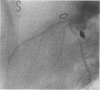
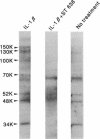
We recently demonstrated that chronic treatment with IL-1 beta induces coronary arteriosclerotic changes and vasospastic responses to autacoids in pigs in vivo and that those responses are importantly mediated by PDGF. The receptors for PDGF and other major growth factors are known to have tyrosine kinase activity. We therefore investigated the effects of a selective tyrosine kinase inhibitor, ST 638, on those responses induced by IL-1 beta in our swine model. Intimal thickening and coronary vasospastic responses to serotonin and histamine were induced at the site of the coronary artery where IL-1 beta was chronically and locally applied. These responses were significantly suppressed in a dose-dependent manner by cotreatment with ST 638. In addition, ST 494, which is an inactive form of ST 638, did not inhibit those responses. The treatment with ST 638 alone did not affect the coronary vasoconstricting responses to the autacoids. Immunoblotting using an antibody to phosphotyrosines confirmed the inhibitory effects of ST 638 on the tyrosine phosphorylations induced by IL-1 beta. These results thus suggest that tyrosine kinase activation may play an important role in mediating the effects of IL-1 beta, while also suggesting that ST 638 has an inhibitory effect on the arteriosclerotic changes and vasospastic responses to autacoids in our swine model in vivo.
Full text
PDF

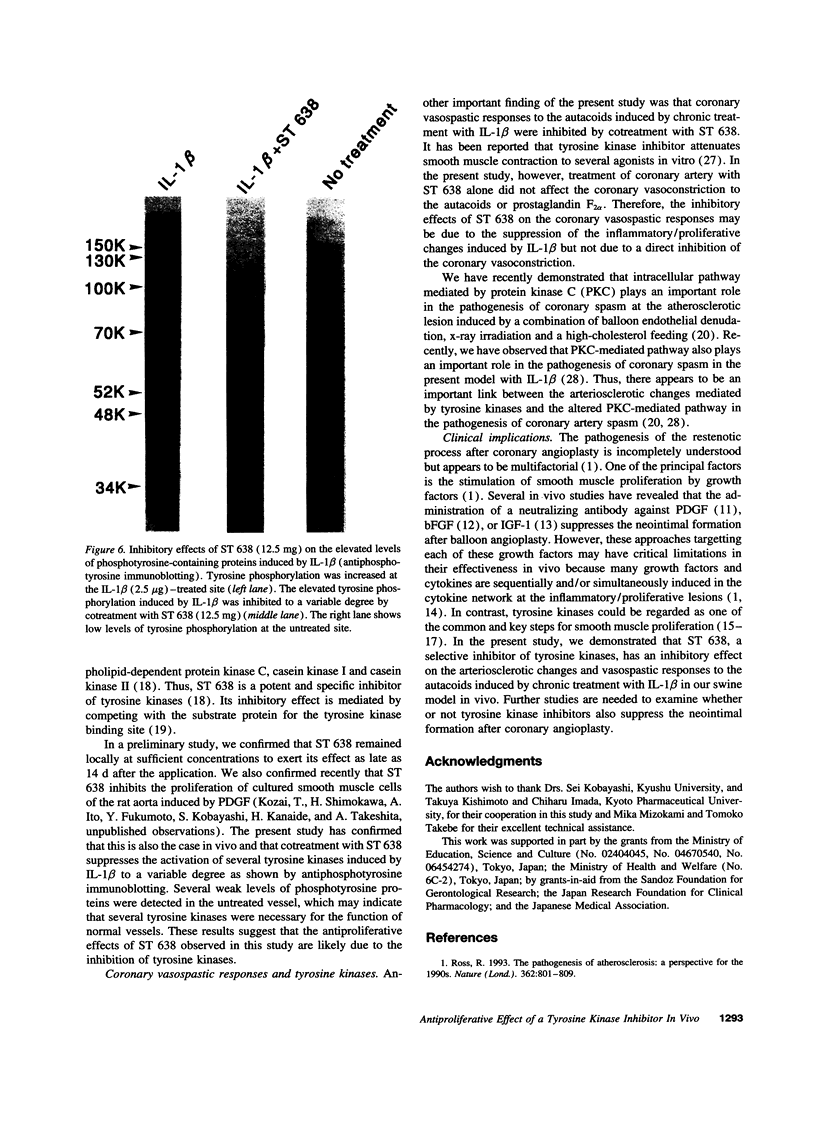

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banskota N. K., Taub R., Zellner K., Olsen P., King G. L. Characterization of induction of protooncogene c-myc and cellular growth in human vascular smooth muscle cells by insulin and IGF-I. Diabetes. 1989 Jan;38(1):123–129. doi: 10.2337/diab.38.1.123. [DOI] [PubMed] [Google Scholar]
- Di Salvo J., Steusloff A., Semenchuk L., Satoh S., Kolquist K., Pfitzer G. Tyrosine kinase inhibitors suppress agonist-induced contraction in smooth muscle. Biochem Biophys Res Commun. 1993 Feb 15;190(3):968–974. doi: 10.1006/bbrc.1993.1144. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Egashira K., Tomoike H., Yamamoto Y., Yamada A., Hayashi Y., Nakamura M. Histamine-induced coronary spasm in regions of intimal thickening in miniature pigs: roles of serum cholesterol and spontaneous or induced intimal thickening. Circulation. 1986 Oct;74(4):826–837. doi: 10.1161/01.cir.74.4.826. [DOI] [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Chen W. S., Lazar C. S., Walton G. M., Zokas L. M., Rosenfeld M. G., Gill G. N. Ligand-induced endocytosis of the EGF receptor is blocked by mutational inactivation and by microinjection of anti-phosphotyrosine antibodies. Cell. 1988 Mar 11;52(5):675–684. doi: 10.1016/0092-8674(88)90405-9. [DOI] [PubMed] [Google Scholar]
- Grant M. B., Wargovich T. J., Ellis E. A., Caballero S., Mansour M., Pepine C. J. Localization of insulin-like growth factor I and inhibition of coronary smooth muscle cell growth by somatostatin analogues in human coronary smooth muscle cells. A potential treatment for restenosis? Circulation. 1994 Apr;89(4):1511–1517. doi: 10.1161/01.cir.89.4.1511. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Seifert P. S., Stemme S. Immune mechanisms in atherosclerosis. Arteriosclerosis. 1989 Sep-Oct;9(5):567–578. doi: 10.1161/01.atv.9.5.567. [DOI] [PubMed] [Google Scholar]
- Ikeda U., Ikeda M., Oohara T., Kano S., Yaginuma T. Mitogenic action of interleukin-1 alpha on vascular smooth muscle cells mediated by PDGF. Atherosclerosis. 1990 Oct;84(2-3):183–188. doi: 10.1016/0021-9150(90)90089-2. [DOI] [PubMed] [Google Scholar]
- Ito A., Shimokawa H., Nakaike R., Fukai T., Sakata M., Takayanagi T., Egashira K., Takeshita A. Role of protein kinase C-mediated pathway in the pathogenesis of coronary artery spasm in a swine model. Circulation. 1994 Nov;90(5):2425–2431. doi: 10.1161/01.cir.90.5.2425. [DOI] [PubMed] [Google Scholar]
- Lenfant C. NHLBI funding policies. Enhancing stability, predictability, and cost control. Circulation. 1994 Jul;90(1):1–1. doi: 10.1161/01.cir.90.1.1. [DOI] [PubMed] [Google Scholar]
- Libby P., Warner S. J., Friedman G. B. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest. 1988 Feb;81(2):487–498. doi: 10.1172/JCI113346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner V., Reidy M. A. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer C. F., Sajuthi D., Tulli H., Williams J. K. Synthesis of IL-1 alpha and IL-1 beta by arterial cells in atherosclerosis. Am J Pathol. 1991 Apr;138(4):951–960. [PMC free article] [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Owada M. K., Iwamoto M., Koike T., Kato Y. Effects of vanadate on tyrosine phosphorylation and the pattern of glycosaminoglycan synthesis in rabbit chondrocytes in culture. J Cell Physiol. 1989 Mar;138(3):484–492. doi: 10.1002/jcp.1041380307. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Shimokawa H., Tomoike H., Nabeyama S., Yamamoto H., Araki H., Nakamura M., Ishii Y., Tanaka K. Coronary artery spasm induced in atherosclerotic miniature swine. Science. 1983 Aug 5;221(4610):560–562. doi: 10.1126/science.6408736. [DOI] [PubMed] [Google Scholar]
- Shimokawa H., Tomoike H., Nabeyama S., Yamamoto H., Ishii Y., Tanaka K., Nakamura M. Coronary artery spasm induced in miniature swine: angiographic evidence and relation to coronary atherosclerosis. Am Heart J. 1985 Aug;110(2):300–310. doi: 10.1016/0002-8703(85)90148-6. [DOI] [PubMed] [Google Scholar]
- Shiraishi T., Domoto T., Imai N., Shimada Y., Watanabe K. Specific inhibitors of tyrosine-specific protein kinase, synthetic 4-hydroxycinnamamide derivatives. Biochem Biophys Res Commun. 1987 Aug 31;147(1):322–328. doi: 10.1016/s0006-291x(87)80124-9. [DOI] [PubMed] [Google Scholar]
- Shiraishi T., Owada M. K., Tatsuka M., Yamashita T., Watanabe K., Kakunaga T. Specific inhibitors of tyrosine-specific protein kinases: properties of 4-hydroxycinnamamide derivatives in vitro. Cancer Res. 1989 May 1;49(9):2374–2378. [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Winkles J. A., Friesel R., Burgess W. H., Howk R., Mehlman T., Weinstein R., Maciag T. Human vascular smooth muscle cells both express and respond to heparin-binding growth factor I (endothelial cell growth factor). Proc Natl Acad Sci U S A. 1987 Oct;84(20):7124–7128. doi: 10.1073/pnas.84.20.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]